Department of Endocrinology, Diabetoloy and Metabolism, Antwerp University Hospital, Antwerp, Belgium
luc.van.gaal@uza.be
Jean-Pierre Després, PhD, FAHA
Québec Heart Institute, Hôpital Laval Research Centre, Québec, QC, Canada, Division of Kinesiology, Department of Social and Preventive Medicine, Université Laval, Québec, QC, Canada
jean-pierre.despres@crhl.ulaval.ca


Introduction
Key Points
-
Abdominal obesity is the high-risk form of obesity.
-
CB1 receptor antagonism can induce weight loss, loss of abdominal fat and improvements in the cardiometabolic risk profile.
-
CB1 receptor antagonists have been shown to decrease intra-abdominal (visceral) and liver fat.
-
Developing the "right drug for the right patient" is an important challenge inherent to compounds that are labelled "weight loss drugs".
-
Whether reducing abdominal obesity can reduce the risk of cardiovascular disease still remains to be determined.
The health hazards of abdominal obesity were documented several decades ago when, in 1947, a French physician by the name of Dr. Jean Vague published in Presse Médicale the results of his clinical observations on the "android" type of obesity ("apple shape") [1]. Vague was the first to suggest that android obesity was the high-risk form of obesity. In contrast, he proposed that "gynoid" obesity (often found in women) was rather benign [1]. Thus, Vague was the first to foresee the importance of upper body, abdominal obesity as a phenotype frequently observed in individuals with cardiovascular disease, type 2 diabetes and hypertension. Results from epidemiological studies that began to be published in the early eighties confirmed the increased risk of adverse cardiovascular outcomes associated with such a form of overweight/obesity. Most of these studies assessed the absolute or relative amount of abdominal fat using crude anthropometric indices such as waist circumference or the waist-to-hip circumference ratio [2-6]. Very recently, the importance of abdominal obesity beyond overall general adiposity as a risk factor for total mortality has been confirmed in the largest prospective study ever conducted on the topic. Results of the EPIC study provided robust evidence that waist circumference predicted mortality beyond body mass index [7]. Studies that have directly measured abdominal fat using imaging techniques such as computed tomography have demonstrated that among abdominally obese individuals, those characterized by a selective excess of intra-abdominal (visceral) fat accumulation have the most atherogenic and diabetogenic metabolic profile (often referred to as the metabolic syndrome) compared to subjects with a selective excess of subcutaneous fat [8-10]. In addition, intra-abdominal fat - as a reflection of overall ectopic fat - may be the link between obesity and cardiovascular disease [11].
State of the Art
 [Click to enlarge]
[Click to enlarge]
As abdominal obesity is an emerging modifiable risk factor for type 2 diabetes and cardiovascular disease, a pharmacological approach targeting the excess abdominal fat depot (which most of the time accompanies features of the metabolic syndrome) could be relevant to optimally reduce the cardiovascular disease risk of patients with intra-abdominal obesity. In this regard, the evidence of an overactivation of the endocannabinoid system (ECS) in obesity, particularly abdominal obesity [12-14], and the published results of the phase III program (Rimonabant In Obesity; RIO) to be conducted with the first CB1 blocker developed, rimonabant, may open new possibilities for targeting abdominal obesity and related abnormalities [15-18]. Rimonabant works centrally to reduce food in-take through antagonism of the cannabinoid receptor (CB1), but there is now evidence that it also acts peripherally in key tissues involved in carbohydrate and lipid metabolism such as the liver and adipose tissue [19-22]. For instance, CB1 blockade with rimonabant has been shown in animals to reduce liver lipogenesis and to stimulate adiponectin gene expression and protein secretion by fat cells [19, 22]. These findings are particularly relevant for the management of the metabolic ab-normalities of intra-abdominal obesity.
Because of the designs requested by regulatory authorities, initial studies with rimonabant have mainly focused on weight loss and on its effect on cardiometabolic risk factors in patients selected only on the basis of their excess body weight. However, the RIO-Lipids study was specifically designed to test the effect of rimonabant in higher-risk patients: those who were not only overweight/obese (body mass index: 27-40 kg/m2) but who also had an atherogenic dyslipidemia (triglyceride levels between 1.7-7.9 mmol/l and/or cholesterol/HDL cholesterol >5 for men or 4.5 for women) [15]. As for all four phase III studies with rimonabant, patients of the RIO-Lipids trial were asked to reduce their caloric intake by 600 kcal/day during a 4-week run-in period, which they did as they lost about 2 kg of body weight and their waist circumference was reduced by 2 cm. After the run-in period, the baseline characteristics of these dyslipidemic patients were assessed and they were then randomized and exposed either to placebo (n=342) or treatment with rimonabant 5 mg (n=345) or 20 mg (n=346) daily for 12 months. By the end of the study, patients treated with rimonabant 20 mg had a significantly greater body weight loss compared with the placebo group; this was accompanied by a significantly greater decrease in waist circumference. In addition, this substantial loss of abdominal fat was, as expected, accompanied by significant improvements in the plasma lipoprotein-lipid profile, which included a reduction in triglycerides (p<0.001) and an increase in HDL cholesterol levels (p<0.001) among patients treated with rimonabant 20 mg. Although there was no change in LDL cholesterol levels with rimonabant therapy, the group treated with rimonabant 20 mg showed an increase in LDL particle size (p=0.008) relative to the placebo group, whereas the proportion of small LDL particles decreased compared to the placebo group (p=0.007). In addition, plasma adiponectin levels increased by 58% (p<0.001) over baseline in the rimonabant 20 mg group, and this difference could not be entirely explained by weight loss. For instance, patients in the placebo group who had a ?10% weight loss had an increase in adiponectin levels of slightly >2 µg/ml whereas patients treated with rimonabant had an increase in adiponectin levels of >3 µg/ml. These results provided the first evidence in a clinical trial that CB1 blockade with rimonabant could have a direct effect on the production of adiponectin by adipose tissue beyond what could be explained by weight loss. Thus, this peripheral effect of rimonabant on adipose tissue metabolism could help explain, at least partly, the drug's well documented effect on cardiometabolic risk markers beyond what can be explained by weight loss, a consistent finding in the phase III RIO program.
One of the four phase III studies with rimonabant (RIO-Diabetes) was performed in over-weight/obese patients with type 2 diabetes who were treated either by sulphonylurea (about 1/3) or with metformin (about 2/3) therapy [17]. In addition to confirming the robust effect of rimonabant on plasma lipids and some other markers of cardiometabolic risk, the study revealed that CB1 antagonism with rimonabant could significantly improve glycemic control (HbA1c levels) beyond the effect mediated by weight loss. Such a glucose-lowering effect of rimonabant was found irrespective of background anti-diabetic therapy. A recent study (SERENADE) has also confirmed the cardiometabolic benefits and glucose-lowering effects of rimonabant in drug-naive patients with type 2 diabetes [23]. As type 2 diabetes is the ultimate manifestation of intra-abdominal obesity and of ectopic fat deposition, these effects of rimonabant on markers of abdominal obesity, glycemic control and cardiometabolic risk variables make this drug an interesting option for the global management of patients with type 2 diabetes.
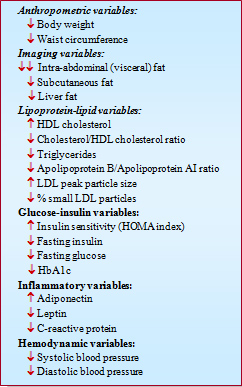
The results of published studies with rimonabant are quite consistent and indicate that rimonabant 20 mg/day produces a significant decrease in body weight as well as a substantial mobilization of abdominal adipose tissue as indicated by a considerable reduction in waist circumference. Moreover, these benefits were found to be maintained over two years in the RIO-Europe trial [24]. Overall, these results suggest that rimonabant therapy could be useful for the management of clustering cardiovascular disease risk factors in high-risk abdominally obese patients through its marked effects on both abdominal adiposity and related metabolic risk factors. In this regard, a recent 1-year imaging trial (ADAGIO-Lipids) has confirmed that rimonabant can induce a significant loss of both intra-abdominal and liver fat [25]. Key cardiometabolic effects of rimonabant are summarized in the Table.
Safety
Antagonism of the ECS clearly produces significant improvements in several markers of cardiometabolic risk. Of course such benefits have to be weighed against the side effects of the drug. Main side effects of the drug have been nausea, dizziness, some gastrointestinal side effects as well as anxiety, mood changes and depression symptoms [26]. Regarding the latter, further analyses from pooled studies as well as more recent trials (such as STRADIVARIUS) have indicated that although the relative risk of depression associated with rimonabant was about 1.7, the absolute risk was largely dependent upon past/present history of depression [27]. On that basis, although regulatory authorities had recommended that rimonabant should not be prescribed in patients with a history of depression, the challenge of ensuring that the right patient is treated with this CB1 antagonist has led the European Medicines Agency (EMEA) to recommend the withdrawal of the drug from the market until further evidence of a favourable benefit/risk ratio becomes available.
Futures Studies/Perspectives
Based on the results published or available with rimonabant, we would like to propose that the best patient for rimonabant therapy is an abdominally obese, insulin-resistant patient with an atherogenic dyslipidemia or an abdominally obese patient with type 2 diabetes. Of course, these two categories of high-risk patients should exclude those for whom there is evidence of past depression episodes or susceptibility to depression. Whether it will ever be possible to develop proper treatment algorithms to make sure that the right patient is treated with rimonabant is uncertain at this stage. However, the discovery of the ECS and of its profound impact on body fat distribution, ectopic fat deposition and carbohydrate and lipid metabolism has been a remarkable breakthrough. It is hoped that this body of knowledge will be properly used to treat the right patient with the right drug.
References
- Vague J. La différenciation sexuelle: facteur déterminant des formes de l'obesité. Presse Med 1947; 339-40.
- Larsson B, Svardsudd K, Welin L, et al. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. BMJ (Clin Res Ed) 1984; 288: 1401-4.
- Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA 1998; 280: 1843-8.
- Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005; 366: 1640-9.
- Canoy D, Boekholdt SM, Wareham N, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation 2007; 116: 2933-43.
- Li TY, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation 2006; 113: 499-506.
- Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008; 359: 2105-20.
- Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med 2006; 38: 52-63.
- Després JP and Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881-7.
- Després JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008; 28: 1039-49.
- Van Gaal LF, Mertens IL and De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444: 875-80.
- Côté M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007; 31: 692-9.
- Engeli S, Bohnke J, Feldpausch M, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005; 54: 2838-43.
- Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 2006; 91: 3171-80.
- Després JP, Golay A and Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005; 353: 2121-34.
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 2006; 295: 761-75.
- Scheen AJ, Finer N, Hollander P, et al. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 2006; 368: 1660-72.
- Van Gaal LF, Rissanen AM, Scheen AJ, et al. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005; 365: 1389-97.
- Bensaid M, Gary-Bobo M, Esclangon A, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 2003; 63: 908-14.
- Di Marzo V, Bifulco M and De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 2004; 3: 771-84.
- Jbilo O, Ravinet-Trillou C, Arnone M, et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. Faseb J 2005; 19: 1567-9.
- Osei-Hyiaman D, DePetrillo M, Pacher P, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005; 115: 1298-305.
- Rosenstock J, Hollander P, Chevalier S, et al. SERENADE: the Study Evaluating Rimonabant Efficacy in Drug-naive Diabetic Patients: effects of monotherapy with rimonabant, the first selective CB1 receptor antagonist, on glycemic control, body weight, and lipid profile in drug-naive type 2 diabetes. Diabetes Care 2008; 31: 2169-76.
- Van Gaal LF, Scheen AJ, Rissanen AM, et al. Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J 2008; 29: 1761-71.
- Després JP, Ross R, Boka G, et al. Rimonabant reduces both intra-abdominal adiposity and liver fat and improves cardiometabolic risk factors: the ADAGIO-Lipids trial. Presented at the late breaking session of the 77th European Atherosclerosis Society meeting, Istanbul, Turkey, 2008.
- Van Gaal L, Pi-Sunyer X, Després JP, et al. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care 2008; 31 Suppl 2: S229-40.
- Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA 2008; 299: 1547-60.



