Ariane Mallat, MD, PhD Inserm U841, Hôpital Henri Mondor, Créteil,
France
Sophie.Lotersztajn@inserm.fr

Introduction
Key Points
- Chronic liver disease is responsible for about 800,000 deaths a year due to cirrhosis and its complications.
- Viral hepatitis, chronic alcohol consumption, and non-alcoholic fatty liver disease are the most common causes of liver disease worldwide. All these conditions generate liver injury and inflammation, thereby activating liver fibrogenesis, which can progress to cirrhosis and the life-threatening complications of liver failure and portal hypertension, as well as to incident hepatocellular carcinoma.
- The ECS is highly up-regulated during liver injury.
- CB1 receptors promote fatty liver via direct activation of CB1 receptors expressed in hepatocytes.
- CB1 receptors are profibrogenic.
- CB1 receptors contribute to the hemodynamic complications of cirrhosis.
- Beneficial effects of CB1 antagonists are expected in patients with non-alcoholic or alcoholic fatty liver disease, at multiple steps of liver disease progression.
- The development of peripherally restricted CB1 antagonists will constitute a major challenge within the next few years.
- CB2 receptors also participate in the pathogenesis of non- alcoholic fatty liver disease via a pathway distinct from that activated by CB1 receptors: CB2 receptors indirectly enhance metabolic steatosis, following increased obesity-associated fat inflammation and insulin resistance.
- CB2 receptors are antifibrogenic.
- Potential therapeutic indications of CB2-specific molecules are expected but will require additional preclinical studies in order to precisely define the conditions associated with CB2-dependent pro or anti-inflammatory effects.
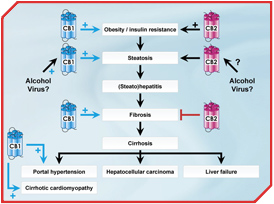
Chronic liver disease is responsible for about 800,000 deaths a year due to cirrhosis and its complications. The most common causes of liver disease worldwide are viral hepatitis, chronic alcohol consumption, and non-alcoholic fatty liver disease (NAFLD) associated with the metabolic syndrome. All these conditions generate liver injury and inflammation, thereby activating liver fibrogenesis. Progression of fibrosis leads to cirrhosis and the life-threatening complications of liver failure and portal hypertension, as well as to incident hepatocellular carcinoma [1].
Accumulating evidence indicates that the endocannabinoid system (ECS) plays a crucial role in the pathophysiology of liver diseases, both as a key player in hepatic injury and as a mediator of cirrhosis complications. Indeed, CB1 and CB2 receptors have emerged as mediators of non-alcoholic and alcoholic fatty liver disease and regulate hepatic inflammation, liver fibrosis, and complications of cirrhosis, including cirrhotic portal hypertension and cirrhotic cardiomyopathy [2, 3].
State of the Art
The normal liver produces endocannabinoids [2, 4], originating from both hepatocytes and non-parenchymal cells [5], and expresses low levels of cannabinoid receptors. In contrast, the ECS is up-regulated during liver injury and affects several physiopathological processes associated with acute or chronic liver disease.
The ECS and fatty liver disease
NAFLD is linked to the metabolic syndrome and is a rising cause of liver injury in Western countries. NAFLD shares common pathologic features and pathophysiological mechanisms with alcoholic fatty liver disease. Both diseases can present as steatosis but may evolve towards alcoholic or non-alcoholic steatohepatitis, when associated with liver inflammation and hepatocyte injury, that promotes liver fibrogenesis, with a 20% risk of cirrhosis after 10 to 20 years [6].
 [Click to enlarge]
[Click to enlarge]
Recent studies have demonstrated the major role of CB1 receptors in fatty liver disease. Hence, hepatic endocannabinoids are overproduced and hepatocyte CB1 receptors are up-regulated in response to high-fat diet or chronic alcohol feeding [7-9]. Moreover, CB1 receptor-deficient mice do not develop steatosis, and high fat diet or ethanol-induced fatty liver is prevented by rimonabant treatment [7-9]. Interestingly, mice with hepatocyte-specific deletion of CB1 receptors are resistant to fatty liver, thereby supporting a direct role of hepatic CB1 receptors in this process [8]. This data is reinforced by clinical evidence showing that daily cannabis use is an independent predictor of steatosis severity in patients with chronic hepatitis C [10].
CB2 receptors also contribute to the pathogenesis of NAFLD. Indeed, mice deficient in CB2 receptors are more resistant to high fat diet-induced obesity than wild type animals. Moreover, CB2 antagonism improves insulin sensitivity and blunts hepatic steatosis following inhibition of obesity-associated inflammation in the adipose tissue [4]. These results demonstrate that both CB1 and CB2 play a role in the pathogenesis of NAFLD via distinct pathways. Hepatic inflammation is prominently involved in the process.
The ECS and liver fibrogenesis
The frequent inability to eradicate the cause of chronic liver disease warrants the development of liver-specific antifibrotic strategies that generally aim at inhibiting the accumulation of liver fibrogenic cells and/or reducing extracellular matrix accumulation. In addition, inhibition of parenchymal injury or reduction of liver inflammation has also been shown to have some beneficial antifibrogenic effects [1]. However, despite encouraging experimental results, proof of efficacy of potential antifibrogenic molecules in a clinical setting is currently lacking.
Recent studies have shown that the ECS may be a crucial regulator of liver fibrogenesis. CB1 and CB2 receptors are up-regulated in the cirrhotic human liver, predominantly in liver fibrogenic cells. Moreover, endogenous activation of CB2 receptors limits progression of experimental liver fibrosis by reducing accumulation of liver fibrogenic cells, thereby demonstrating the antifibrogenic properties of CB2 receptors [11]. Interestingly, CB2 receptors also display anti-inflammatory properties during liver ischemia-reperfusion injury [5]. However, whether CB2 agonists may, in addition to directly limiting hepatic fibrogenic cell accumulation, also indirectly regulate fibrogenesis by inhibiting the inflammatory response to chronic liver injury remains to be determined.
In contrast to CB2 receptors, CB1 is profibrogenic in the liver. Administration of rimonabant or genetic inactivation of CB1 receptors inhibits fibrosis progression in three models of chronic liver injury by a mechanism involving reduced proliferation and increased apoptosis of liver fibrogenic cells [12]. Moreover, daily cannabis use is an independent predictor of fibrosis severity in patients with chronic hepatitis C, suggesting that CB1 signalling predominates over CB2 in these patients [13].
These findings unravel CB1 and CB2 receptors as potential novel targets for antifibrogenic therapy during chronic liver diseases and suggest that combined therapy with selective CB1 antagonists and/or CB2 agonists might open novel perspectives for the treatment of liver fibrosis.
Endocannabinoids as mediators of vascular and cardiac abnormalities in cirrhosis
The ECS contributes to the hemodynamic alterations associated with cirrhosis. Indeed, endocannabinoids trigger vasorelaxing effects, and CB1 receptors contribute to the pathogenesis of portal hypertension via enhanced mesenteric vasodilation [14, 15]. Moreover, the cardiac expression of the ECS is increased in experimental models of cirrhosis and is associated with a CB1-dependent impairment of cardiac contractility, demonstrating the role of the CB1 receptor in the development of cirrhotic cardiomyopathy [16, 17].
Priorities for Future Studies
The ECS is increasingly incriminated in several pathophysiological aspects associated with chronic liver disease progression (Figure). Steatogenic and profibrogenic properties of CB1 receptors and their harmful impact on hemodynamic complications of cirrhosis suggest that CB1 receptors trigger several deleterious effects that may enhance progression of chronic liver disease to cirrhosis and its complications. Beneficial effects of CB1 antagonists are therefore expected in patients with non-alcoholic or alcoholic fatty liver disease, at multiple steps of disease progression. However, the increased incidence of anxiety and depression in obese patients treated with rimonabant has led to its recent withdrawal by the European Medicines Agency (EMEA). The development of peripherally restricted CB1 antagonists will therefore constitute a major challenge within the next few years. CB2 receptors play a key role in the regulation of the liver inflammatory response [18]. These findings may open novel therapeutic perspectives on clinical development of CB2 specific molecules. However, potential therapeutic indications will require additional preclinical studies in order to precisely define the conditions associated with CB2-dependent pro- or anti-inflammatory effects.
References
- Lotersztajn S, Julien B, Teixeira-Clerc F, et al. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol 2005; 45: 605-28.
- Mallat A and Lotersztajn S. Endocannabinoids as novel mediators of liver diseases. J Endocrinol Invest 2006; 29: 58-65.
- Mallat A and Lotersztajn S. Endocannabinoids and liver disease. I. Endocannabinoids and their receptors in the liver. Am J Physiol Gastrointest Liver Physiol 2008; 294: G9-G12.
- Deveaux V, Ichigotani Y, Teixeira-Clerc F, et al. CB2 receptor antagonism reduces diet-induced obesity, insulin resistance and hepatic steatosis. Hepatology 2007; 46: 308A.
- Batkai S, Osei-Hyiaman D, Pan H, et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. Faseb J 2007; 21: 1788-800.
- Tilg H and Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med 2000; 343: 1467-76.
- Gary-Bobo M, Elachouri G, Gallas JF, et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology 2007; 46: 122-9.
- Jeong WI, Osei-Hyiaman D, Park O, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab 2008; 7: 227-35.
- Osei-Hyiaman D, DePetrillo M, Pacher P, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005; 115: 1298-305.
- Hezode C, Zafrani ES, Roudot-Thoraval F, et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology 2008; 134: 432-9.
- Julien B, Grenard P, Teixeira-Clerc F, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005; 128: 742-55.
- Teixeira-Clerc F, Julien B, Grenard P, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 2006; 12: 671-6.
- Hezode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology 2005; 42: 63-71.
- Batkai S, Jarai Z, Wagner JA, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med 2001; 7: 827-32.
- Ros J, Claria J, To-Figueras J, et al. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology 2002; 122: 85-93.
- Batkai S, Mukhopadhyay P, Harvey-White J, et al. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol 2007; 293: H1689-95.
- Gaskari SA, Liu H, Moezi L, et al. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile ductligated rats. Br J Pharmacol 2005; 146: 315-23.
- Lotersztajn S, Teixeira-Clerc F, Julien B, et al. CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol 2008; 153: 286-9.



