Laboratoire d'Études Moléculaires des Valvulopathies (LEMV), Groupe de Recherche en Valvulopathies (GRV), Centre de recherche de l'Institut universitaire de cardiologie et de pneumologie de Québec; Department of Surgery, Université Laval, Québec, QC, Canada.
patrick.mathieu@chg.ulaval.ca

Key Points
- The metabolic syndrome is associated with aortic valve calcification and a faster aortic stenosis progression rate.
- Among the different features of the metabolic syndrome, a high proportion of small, dense LDL in blood plasma is associated with aortic valve inflammation along with a clinically faster progression rate.
- Following an aortic valve replacement, the metabolic syndrome increases the risk of bioprostheses structural degeneration.
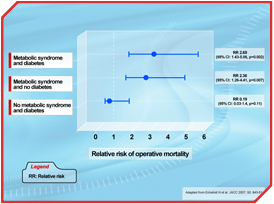
- The metabolic syndrome is a risk factor in cardiac surgery increasing substantially the risk of postoperative death.
- In middle-aged patients, the metabolic syndrome increases the risk of postoperative atrial fibrillation, a common arrhythmia following coronary artery bypass surgery.
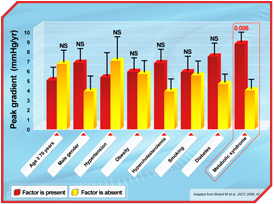
During the 17th century, in the midst of the Elizabethan Era, Sir Walter Raleigh brought tobacco from the New World to England. This marked the start of a new habit: smoking. Unfortunately, this new habit also proved to be a new health risk factor with devastating consequences. Quite later, during the second half of the 20th century, major social changes following the industrial revolution led to the introduction of commercially prepared, highly processed food in the New World, and this new way of life, now sometimes referred to as 'coca-colonization', spread at a startling speed throughout the world [1]. So, while smoking has decreased considerably in numerous developed countries, largely because of national health care policies, obesity has gained ground and is now considered by many authorities to be a major health care problem. As a result, obesity-associated conditions such as diabetes have reached gigantic proportions on a worldwide scale. In light of the research conducted during the last decade or so, it is important to stress from the outset that obesity is a very heterogeneous condition in which body fat distribution largely determines metabolic features [2]. In this regard, intra-abdominal (visceral) obesity has been consistently associated with glucose intolerance, low HDL, hypertriglyceridemia, and hypertension [3]. Intra-abdominal obesity, the most common form of the metabolic syndrome, has been linked to an atherogenic dyslipidemia and thereby to a high risk of coronary artery disease (CAD) [4]. Of special interest is the fact that the metabolic syndrome has recently been shown to be a composite modifiable cardiovascular risk factor, which goes far beyond the so-called traditional risk factors [5]. As a matter of fact, it now appears that the metabolic syndrome is a strong risk factor for the development of aortic valve calcification and the progression rate of aortic stenosis (AS) [6]. AS, long thought of as a passive 'degenerative' disorder, is now considered a highly regulated process akin to atherosclerosis [7]. Studies in the last decade have conclusively shown that AS valves are infiltrated with oxidized-LDL and inflammatory cells, whereby the calcification is triggered and perpetuated. In vitro studies with aortic valvular interstitial cells have indicated that, when grown in appropriate conditions, interstitial cells may change their phenotype toward bone-like cells [8]. Since AS is an active and cellular process that shares some similarities with atherosclerosis, specifically the histological picture and risk factors, statins were seen as a way to treat AS in order to stop or lower the hemodynamic progression rate. However, this conception has been questioned recently with the publication of the first two randomized studies with statins in AS [9, 10]. In the two trials, despite a dramatic reduction of LDL cholesterol of at least 50%, aortic valve calcification as well as the hemodynamic progression rate remained unaffected by the lipid-lowering strategy. Recently, our group has demonstrated that among different clinical risk factors, the metabolic syndrome is a strong and independent risk factor for AS progression (Figure 1). More recently, a study has demonstrated that the small, dense LDL phenotype associated with intra-abdominal obesity is associated with aortic valve accumulation of oxidized-LDL and, perhaps more importantly, with the progression rate of AS [11]. This study has contributed, at least in part, to explain the inefficacy of statins in AS. It should be emphasized that the small, dense LDL phenotype is often associated with a near-normal or normal LDL level, and that statins have at best only a modest effect on the proportion of small, dense LDL in circulation.
 [Click to enlarge]
[Click to enlarge]
Thus, it is quite likely that the metabolic perturbations associated with intra-abdominal obesity, such as the high proportion of small, dense LDL, determine valve accumulation of oxidized lipids and thereby influence the mineralization process as well as the clinical activity of this disorder.
In the absence of effective medical treatments and possibly owing to the epidemic proportions obesity has reached, there has been a recent increase in the prevalence of severe AS and a considerable influx of patients to surgery to replace these end-stage diseased valves. Globally, about half of these aortic valve replacements are done with bioprostheses, made either of porcine or bovine tissues. Although biosprosthetic valves have numerous advantages, their 'Achilles heel' is their propensity to calcify and become dysfunctional. While for many years this degenerative process was thought to be related to pre-implant fixation technique, recent investigations suggest that metabolic risk factors could also be involved. For instance, among different factors, the metabolic syndrome has been associated with early hemodynamic dysfunction of bioprostheses [12]. It is suspected that there are numerous mechanisms underpinning bioprosthesis dysfunction, including the possibility that some metabolic features of intra-abdominal obesity contribute to the inflammation of bioprostheses and matrix degradation.
 [Click to enlarge]
[Click to enlarge]
While considerable interest has been devoted to the mechanism by which excess intra-abdominal fat contributes to metabolic perturbations and increased cardiovascular risk, it is only recently that attention has been paid to the mechanisms controlling the distribution of excess energy in different fat depots. It is now apparent that the biology of adipocytes is quite complex and that adipose tissue acts as an endocrine organ having intricate links with the metabolism, the brain, and the cardiovascular system. It has been elegantly shown that among other mechanisms, leptin, an adipokine with many functions, plays a crucial role in the partition of lipids and prevents ectopic fat accumulation [15]. According to the lipotoxic theory, accumulation of fat in organs, for which it is not the primary function, may contribute to cellular injury through the production of ceramides. New imaging technologies, such as magnetic resonance imaging, as well as direct surgical observation of excessive epicardial fat accumulation have resurrected an old observation in the realm of modern scientific inquiry. In the 18th century, physicians had already observed the 'fatty heart' at autopsy and had suspected a possible link between this condition and sudden death. More recently, magnetic resonance imaging studies have shown positive relationships between epicardial fat accumulation, intra-abdominal obesity, and a decreased regional systolic shortening of the myocardium [16]. Considering the common embryologic origin of epicardial and intra-abdominal fat, it is likely that epicardial fat is pro-inflammatory. It then follows that owing to the production of cytokines, epicardial fat may have an impact on the development of atherosclerosis. It should be pointed out that contrary to epicardial coronary arteries, intra-myocardial arteries are often in pristine condition without any sign of atherosclerosis. This latter observation may then lend weight to the role of epicardial fat, which is intimately related to epicardial arteries, as a potential contributor to atherosclerosis and/or plaque biological activity. Though the role of epicardial fat in cardiovascular pathologies is still ill-defined, it should be acknowledged that it deserves attention and further mechanistic studies, given its potential impact.
In light of the above discussion, excessive accumulation of ectopic/intra-abdominal fat appears to be a significant cardiovascular risk factor. Studies have therefore brought heart valve diseases, arrhythmia, and operative risk into the realm of cardiometabolic risk. With the identification of intra-abdominal obesity's huge impact on cardiovascular risk, and considering the epidemic proportions of obesity, a concerted effort from the scientific community and health care agencies is of paramount importance. Indeed, primary and secondary prevention, as well as identifying key molecular targets of the multi-faceted manifestations of intra-abdominal obesity on cardiovascular pathology, will be necessary to stave off the devastating consequences of our modern lifestyle.
References
- Zimmet P. Globalization, coca-colonization and the chronic disease epidemic: can the Doomsday scenario be averted? J Intern Med 2000; 247: 301-10.
- Mathieu P, Poirier P, Pibarot P, et al. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension 2009; 53: 577-84.
- Després JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008; 28: 1039-49.
- Després JP, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996; 334: 952-7.
- Mathieu P, Pibarot P, Larose E, et al. Visceral obesity and the heart. Int J Biochem Cell Biol 2008; 40: 821-36.
- Briand M, Lemieux I, Dumesnil JG, et al. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol 2006; 47: 2229-36.
- Pibarot P, Dumesnil JG and Mathieu P. [New insight into the treatment of aortic stenosis]. Med Sci (Paris) 2007; 23: 81-7.
- Mathieu P, Voisine P, Pépin A, et al. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis 2005; 14: 353-7.
- Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352: 2389-97.
- Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359: 1343-56.
- Mohty D, Pibarot P, Després JP, et al. Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol 2008; 28: 187-93.
- Briand M, Pibarot P, Després JP, et al. Metabolic syndrome is associated with faster degeneration of bioprosthetic valves. Circulation 2006; 114: I512-7.
- Echahidi N, Pibarot P, Després JP, et al. Metabolic syndrome increases operative mortality in patients undergoing coronary artery bypass grafting surgery. J Am Coll Cardiol 2007; 50: 843-51.
- Echahidi N, Mohty D, Pibarot P, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation 2007; 116: I213-9.
- Unger RH. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie 2005; 87: 57-64.
- McGavock JM, Victor RG, Unger RH, et al. Adiposity of the heart, revisited. Ann Intern Med 2006; 144: 517-24.



