Abdominal Obesity and Type 2 Diabetes
Defining CMR - Epidemiology - Abdominal Obesity vs. Type 2 Diabetes: Beyond Body WeightKey Points
- The fast, simultaneous growth of obesity and type 2 diabetes has led to the coining of the term “diabesity” to illustrate the close relationship between these two diseases.
- A preferential accumulation of abdominal adipose tissue has been linked to glucose intolerance, hyperinsulinemia, and insulin resistance, which are metabolic complications predictive of increased type 2 diabetes risk.
- Using simple tools such as waist circumference to measure visceral adiposity can help assess type 2 diabetes risk independent of relative weight (BMI).
- These findings underscore the need to go beyond simple measurement of total body weight and BMI when evaluating type 2 diabetes risk in patients.
The “Diabesity” Epidemic
As a health hazard, obesity has been linked to numerous metabolic complications such as dyslipidemia, type 2 diabetes, and cardiovascular disease (CVD) [1,2]. The number of overweight or obese individuals is fast increasing worldwide, and this increase has meant a concomitant rise in the prevalence of type 2 diabetes [2,3]. With type 2 diabetes reaching epidemic proportions, the International Diabetes Federation (IDF) has predicted that the number of individuals with diabetes may rise to 700 million by 2045 [4]. The term “diabesity” has been coined [5,6] to emphasize the close relationship between these two diseases. Indeed, obesity and type 2 diabetes frequently occur together, and the vast majority of type 2 diabetic individuals are or have been obese [7,8]. Along with genetic susceptibility, obesity is the most important risk factor for type 2 diabetes [9,10]. The term “diabesity” singles out excess body weight as the major cause of type 2 diabetes [5].
Beyond Excess Body Weight
The strong relationship between obesity and type 2 diabetes does not mean that being overweight or obese will inevitably cause type 2 diabetes. For example, some normal weight individuals may be at high risk of developing type 2 diabetes, whereas many very obese individuals are not insulin resistant [11] and may never develop the disease. Some etiological factors linking excess body fat and type 2 diabetes could explain this phenomenon. Obesity and type 2 diabetes share a number of causative lifestyle factors such as excessive energy intake, a diet high in saturated fat, and a sedentary lifestyle. However, the risk of developing type 2 diabetes may also depend on genetic susceptibility and the distribution of adipose tissue [12].
The possibility of a relationship between body fat distribution and type 2 diabetes was first raised in the mid-forties by French physician Jean Vague [13]. He reported that the complications generally found in obese patients depended more on the location of the excess fat rather than obesity per se [13]. Following this observation, he described the high-risk form of obesity as “android obesity,” a condition commonly found in men and in which adipose tissue accumulates in the trunk. In opposition, he described the accumulation of body fat in the gluteo-femoral region—the common fat pattern of premenopausal women—as “gynoid obesity.” This type of fat distribution rarely leads to common obesity-related complications [14,15]. Since these pioneering observations, many cross-sectional and prospective studies have linked type 2 diabetes to body fat distribution.
Abdominal Obesity: the Diabetogenic Obesity
Several prospective studies have shown that abdominal obesity increases the risk of type 2 diabetes [16,17]. This is due largely to the fact that large amounts of abdominal fat can cause metabolic complications such as glucose intolerance, hyperinsulinemia, and insulin resistance, all of which increase the risk of type 2 diabetes [18,19]. Indeed, individuals with abdominal obesity often have impaired plasma glucose-insulin homeostasis [13,18,20,21]. Excess abdominal adipose tissue has been linked to hyperinsulinemia during the fasting state as well as following an oral glucose load [18,19]. The increased insulin secretion, insulin resistance, and decreased hepatic insulin extraction that are common metabolic complications of abdominal obesity could explain the hyperinsulinemia also found in abdominally obese individuals. Moreover, obese patients with abdominal adipose tissue accumulation are often glucose intolerant despite their hyperinsulinemia, which suggests these subjects are insulin resistant [18,19]. It has been suggested that the relationship between excess abdominal adipose tissue and diabetogenic abnormalities is in part related to the direct release of free fatty acids into the portal vein [22]. These free fatty acids could decrease hepatic clearance of insulin and worsen systemic hyperinsulinemia [23], a precursor to type 2 diabetes. However, other factors such as the many adipokines (interleukin-6, tumour necrosis factor-α, adiponectin) released by adipose tissue might also contribute to the insulin-resistant state observed among individuals with abdominal obesity [24].
Although the mechanism linking abdominal obesity and type 2 diabetes is not fully understood, several studies using anthropometric measurements have reported that upper body obesity—also known as “android obesity”—was more common in diabetic patients than in non-diabetic patients [25,26]. In 1969, Feldman et al. [26] demonstrated in a study of over 7,000 subjects that type 2 diabetic subjects had more total fat mass and that this mass was distributed in the upper body. Another study of some 15,000 women [25] showed that obese women who accumulated body fat in the abdominal area had a tenfold increase in their risk of diabetes compared to non-obese women who accumulated body fat in the gluteo-femoral region.
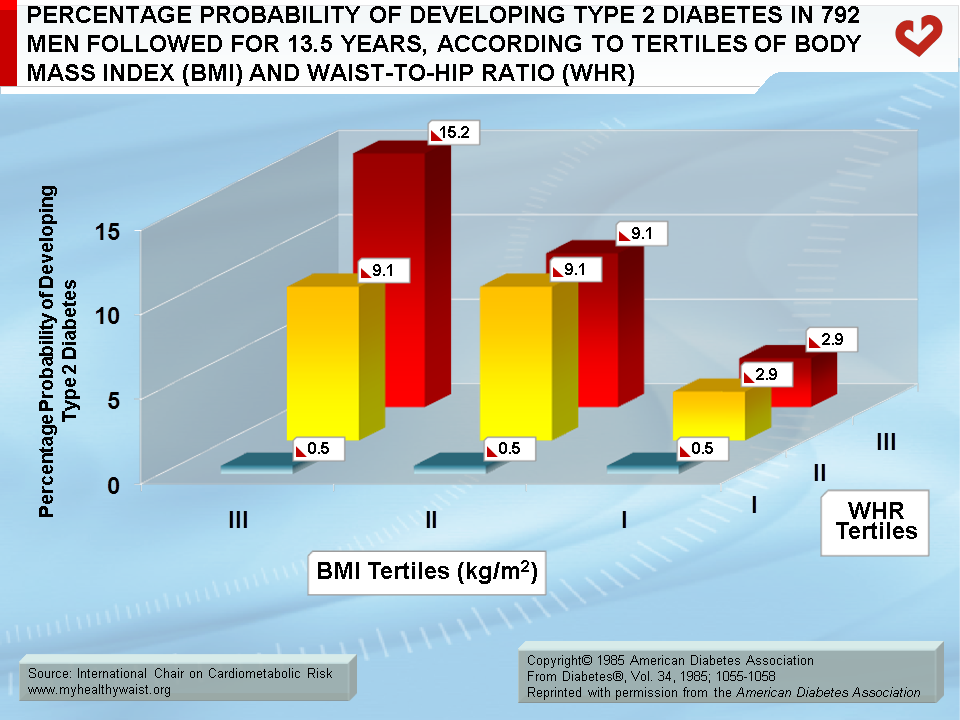
Ohlson et al. [17] conducted the first large prospective study on the link between fat distribution and the incidence of type 2 diabetes. The study involved 792 men selected by year of birth (54 years old) and followed for 13.5 years. The population sample was divided into tertiles according to body mass index (BMI) and waist-to-hip ratio (WHR) as a marker of relative abdominal fat accumulation (Figure). The authors found that the risk of type 2 diabetes rose in step with WHR within each BMI tertile. Even among non-obese men, being in the third WHR tertile was associated with an increased incidence of type 2 diabetes. Moreover, in the lowest WHR tertile, the risk of developing the disease did not increase with BMI. The subgroup with both elevated BMI and WHR were at highest risk of type 2 diabetes. Furthermore, elevated BMI and WHR increased the risk of type 2 diabetes thirtyfold compared to elevated BMI alone. These observations indicate that abdominal adipose tissue measured by WHR is an important marker of diabetes risk even when the degree of overall obesity is taken into account.
The role played by body fat distribution in type 2 diabetes risk has also been investigated in women. In 1989, a prospective study of 1,492 women followed for 12 years reported that WHR independently predicted type 2 diabetes [27]. In the Nurses’ Health Study—a prospective study conducted on a cohort of over 43,000 women free of diabetes and other major chronic diseases at baseline—Carey et al. [28] examined the 8-year incidence of type 2 diabetes among quintiles of baseline BMI and waist circumference values. They found a consistent increase in type 2 diabetes risk as waist circumference increased within each BMI category. They also found that a high BMI value predicted type 2 diabetes, even among women with a low WHR or low waist circumference values. The Nurses’ Health Study therefore yielded solid evidence that using waist circumference to measure abdominal obesity can enhance our ability to predict type 2 diabetes risk beyond what can be provided by BMI. However, BMI should be measured along with waist circumference to properly evaluate type 2 diabetes risk among overweight/obese patients.
A large prospective study by Wang et al. [29] examined a cohort of over 27,000 men followed for 12 years. The study compared the predictive power of waist circumference, WHR, and BMI in diagnosing type 2 diabetes. To assess the independent and additive associations of overall obesity and abdominal adiposity to type 2 diabetes risk, subjects were stratified by BMI categories (30 kg/m2) and further classified by waist circumference (100 cm) and WHR (0.95). Study findings showed that type 2 diabetes risk increased with waist circumference and WHR within each category of baseline BMI, revealing that both overall and abdominal adiposity independently predict type 2 diabetes risk. The study also found that measuring abdominal adiposity using waist circumference and WHR provides a strong indication of type 2 diabetes risk, independent of overall obesity.
Upper Body Fat Distribution: the Importance of Visceral Adipose Tissue
Evidence is mounting in support of the notion that it is not excess body fat but rather high visceral adiposity that increases the risk of type 2 diabetes and CVD [30]. For a given amount of total body fat, individuals with more visceral adipose tissue are at substantially greater risk of being insulin resistant and developing atherogenic and diabetogenic complications [30,31]. It has been shown that visceral adipose tissue is more metabolically active and more closely tied to metabolic abnormalities than subcutaneous adipose tissue [32,33]. In 1987, Fujioka et al. [34] were the first to propose that a preferential accumulation of visceral adipose tissue could explain the deterioration in glucose and lipid metabolism observed in obese patients. They found that subjects with large amounts of visceral adipose tissue had higher fasting plasma triglyceride levels and higher plasma glucose responses following an oral glucose challenge than subjects who had the same total body fat mass (as measured by BMI) but a preferential accumulation of abdominal subcutaneous adipose tissue. Pouliot et al. [35] later quantified the respective contributions of subcutaneous and visceral fat to glucose intolerance and hyperinsulinemia in obese individuals. Two groups of obese patients were carefully matched for the same amount of total body fat but had either low or high amounts of visceral adipose tissue. The authors found that obese individuals with low levels of visceral adipose tissue had normal glucose tolerance when compared to lean controls. However, obese subjects with high levels of visceral adipose tissue showed an increase in their glycemic and insulinemic responses to an oral glucose challenge. Additional studies by Ross et al. [36,37] further explored the relationship between visceral adipose tissue and metabolic risk in men and women. When individuals who were matched for similar abdominal subcutaneous adipose tissue but had different levels of visceral adipose tissue were compared, it was revealed that subjects with high visceral adipose tissue had higher glucose values following an OGTT and lower glucose disposal values compared to subjects with low visceral adipose tissue. These results lend weight to the idea that obese individuals with excess visceral adipose tissue display alterations in indices of plasma glucose-insulin homeostasis and are therefore at increased risk of developing type 2 diabetes.
Similarly, the Insulin Resistance Atherosclerosis Study (IRAS) showed that waist circumference—which is the best anthropometric parameter to crudely assess the absolute amount of visceral fat—was a strong predictor of reduced peripheral insulin action in non-diabetic lean individuals [38]. Wang et al. [29] have also confirmed that waist circumference is a better predictor of type 2 diabetes than WHR or BMI.
In addition, a prospective study by Boyko et al. [39] conducted among Japanese Americans followed for 6 to 10 years examined the relationship between direct measurement of visceral fat via computed tomography and incidence of type 2 diabetes. They found that excess visceral adiposity preceded the disease’s development in Japanese Americans. Visceral adipose tissue was predictive of type 2 diabetes independent of fasting insulin, insulin secretion, glycemia, total and regional adiposity, and family history of diabetes. This study confirms that visceral adipose tissue plays a key role in the development of type 2 diabetes.
In summary, measuring waist circumference makes it easier to identify patients with visceral obesity, insulin resistance, and metabolic complications leading to type 2 diabetes [40]. Unlike overall obesity per se—which can cause moderate metabolic complications—excess visceral adipose tissue has a significant negative effect on indices of plasma glucose-insulin homeostasis that predict an increased risk of type 2 diabetes [41]. Measuring visceral adipose tissue using simple tools such as waist circumference should therefore be a crucial step in assessing type 2 diabetes risk among patients.
References
-
Bray GA, Bouchard C and James WPT, eds. Handbook of obesity. New York: Marcel Dekker. 1998.
PubMed ID:
-
González-Muniesa P, Mártinez-González MA, Hu FB, et al. Obesity. Nat Rev Dis Primers. 2017;3:17034.
PubMed ID: 28617414
-
Ford ES, Williamson DF and Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997; 146: 214-22.
PubMed ID: 9247005
-
International Diabetes Federation. Diabetes facts and figures. https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html.
PubMed ID:
-
Astrup A and Finer N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes Rev 2000; 1: 57-9.
PubMed ID: 12119987
-
Shafrir E. Development and consequences of insulin resistance: lessons from animals with hyperinsulinaemia. Diabetes Metab 1996; 22: 122-31.
PubMed ID: 8792092
-
Stumvoll M, Goldstein BJ and van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005; 365: 1333-46.
PubMed ID: 15823385
-
Halpern A and Mancini MC. Diabesity: are weight loss medications effective? Treat Endocrinol 2005; 4: 65-74.
PubMed ID: 15783244
-
Scheen AJ. Pathophysiology of type 2 diabetes. In: Kuhlman J, Puls W, eds, Handbook of Experimental Pharmacology, Oral Antidiabetics (Springer Verlag: Berlin) 1996; 7-42.
PubMed ID:
-
DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev 1997; 5: 177-269.
PubMed ID:
-
Abbasi F, Brown BW, Jr., Lamendola C, et al. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 2002; 40: 937-43.
PubMed ID: 12225719
-
Vague J and Vague P. Obesity and diabetes. In Alberti KGMM, Krall LP, eds. The Diabetes Annual/4 1988. Amsterdam: Elsevier Science, 1988: 311-38.
PubMed ID:
-
Vague J. La différenciation sexuelle: facteur déterminant des formes de l’obésité. Presse Med 1947; 339-40.
PubMed ID: 18918084
-
Terry RB, Stefanick ML, Haskell WL, et al. Contributions of regional adipose tissue depots tp plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism 1991; 40: 733-40.
PubMed ID: 1870428
-
Pouliot MC, Després JP, Nadeau A, et al. Associations between regional body fat distribution, fasting plasma free fatty acid levels and glucose tolerance in premenopausal women. Int J Obes 1990; 14: 293-302.
PubMed ID: 2361806
-
Kissebah AH, Freedman DS and Peiris AN. Health risks of obesity. Med Clin North Am 1989; 73: 111-38.
PubMed ID: 2643000
-
Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 1985; 34: 1055-8.
PubMed ID: 4043554
-
Bjorntorp P. Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabetes Metab Rev 1988; 4: 615-22.
PubMed ID: 3065014
-
Kissebah AH and Peiris AN. Biology of regional body fat distribution: relationship to non-insulin-dependent diabetes mellitus. Diabetes/Metabolism Reviews 1989; 5: 83-109.
PubMed ID: 2647436
-
Kissebah AH, Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982; 54: 254-60.
PubMed ID: 7033275
-
Krotkiewski M, Bjorntorp P, Sjostrom L, et al. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 1983; 72: 1150-62.
PubMed ID: 6350364
-
Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990; 10: 493-6.
PubMed ID: 2196039
-
Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991; 14: 1132-43.
PubMed ID: 1773700
-
Després JP and Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881-7.
PubMed ID: 17167477
-
Hartz AJ, Rupley DC, Jr., Kalkhoff RD, et al. Relationship of obesity to diabetes: influence of obesity level and body fat distribution. Prev Med 1983; 12: 351-7.
PubMed ID: 6878197
-
Feldman R, Sender AJ and Siegelaub AB. Difference in diabetic and nondiabetic fat distribution patterns by skinfold measurements. Diabetes 1969; 18: 478-86.
PubMed ID: 5795030
-
Lundgren H, Bengtsson C, Blohme G, et al. Adiposity and adipose tissue distribution in relation to incidence of diabetes in women: results from a prospective population study in Gothenburg, Sweden. Int J Obes 1989; 13: 413-23.
PubMed ID: 2793297
-
Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 1997; 145: 614-9.
PubMed ID: 9098178
-
Wang Y, Rimm EB, Stampfer MJ, et al. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005; 81: 555-63.
PubMed ID: 15755822
-
Després JP, Moorjani S, Lupien PJ, et al. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 1990; 10: 497-511.
PubMed ID: 2196040
-
Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med 2006; 38: 52-63.
PubMed ID: 16448989
-
Frayn KN, Karpe F, Fielding BA, et al. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord 2003; 27: 875-88.
PubMed ID: 12861227
-
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21: 697-738.
PubMed ID: 11133069
-
Fujioka S, Matsuzawa Y, Tokunaga K, et al. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 1987; 36: 54-9.
PubMed ID: 3796297
-
Pouliot MC, Després JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 1992; 41: 826-34.
PubMed ID: 1612197
-
Ross R, Aru J, Freeman J, et al. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab 2002; 282: E657-63.
PubMed ID: 11832370
-
Ross R, Freeman J, Hudson R, et al. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab 2002; 87: 5044-51.
PubMed ID: 12414870
-
Alberti KG, Zimmet P and Shaw J. The metabolic syndrome–a new worldwide definition. Lancet 2005; 366: 1059-62.
PubMed ID: 16182882
-
Boyko EJ, Fujimoto WY, Leonetti DL, et al. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 2000; 23: 465-71.
PubMed ID: 10857936
-
Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020; 16: 177-189.
PubMed ID: 32020062
-
Neeland IJ, Ross, R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019; 7: 715-25.
PubMed ID: 31301983
 CLOSE
CLOSE
 CLOSE
CLOSE
González-Muniesa P, Mártinez-González MA, Hu FB, et al. Obesity. Nat Rev Dis Primers. 2017;3:17034.
PubMed ID: 28617414 CLOSE
CLOSE
Ford ES, Williamson DF and Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997; 146: 214-22.
PubMed ID: 9247005 CLOSE
CLOSE
International Diabetes Federation. Diabetes facts and figures. https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html.
PubMed ID: CLOSE
CLOSE
Astrup A and Finer N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes Rev 2000; 1: 57-9.
PubMed ID: 12119987 CLOSE
CLOSE
Shafrir E. Development and consequences of insulin resistance: lessons from animals with hyperinsulinaemia. Diabetes Metab 1996; 22: 122-31.
PubMed ID: 8792092 CLOSE
CLOSE
Stumvoll M, Goldstein BJ and van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005; 365: 1333-46.
PubMed ID: 15823385 CLOSE
CLOSE
Halpern A and Mancini MC. Diabesity: are weight loss medications effective? Treat Endocrinol 2005; 4: 65-74.
PubMed ID: 15783244 CLOSE
CLOSE
 CLOSE
CLOSE
 CLOSE
CLOSE
Abbasi F, Brown BW, Jr., Lamendola C, et al. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 2002; 40: 937-43.
PubMed ID: 12225719 CLOSE
CLOSE
 CLOSE
CLOSE
Vague J. La différenciation sexuelle: facteur déterminant des formes de l’obésité. Presse Med 1947; 339-40.
PubMed ID: 18918084 CLOSE
CLOSE
Terry RB, Stefanick ML, Haskell WL, et al. Contributions of regional adipose tissue depots tp plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism 1991; 40: 733-40.
PubMed ID: 1870428 CLOSE
CLOSE
Pouliot MC, Després JP, Nadeau A, et al. Associations between regional body fat distribution, fasting plasma free fatty acid levels and glucose tolerance in premenopausal women. Int J Obes 1990; 14: 293-302.
PubMed ID: 2361806 CLOSE
CLOSE
Kissebah AH, Freedman DS and Peiris AN. Health risks of obesity. Med Clin North Am 1989; 73: 111-38.
PubMed ID: 2643000 CLOSE
CLOSE
Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 1985; 34: 1055-8.
PubMed ID: 4043554 CLOSE
CLOSE
Bjorntorp P. Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabetes Metab Rev 1988; 4: 615-22.
PubMed ID: 3065014 CLOSE
CLOSE
Kissebah AH and Peiris AN. Biology of regional body fat distribution: relationship to non-insulin-dependent diabetes mellitus. Diabetes/Metabolism Reviews 1989; 5: 83-109.
PubMed ID: 2647436 CLOSE
CLOSE
Kissebah AH, Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982; 54: 254-60.
PubMed ID: 7033275 CLOSE
CLOSE
Krotkiewski M, Bjorntorp P, Sjostrom L, et al. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 1983; 72: 1150-62.
PubMed ID: 6350364 CLOSE
CLOSE
Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990; 10: 493-6.
PubMed ID: 2196039 CLOSE
CLOSE
Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991; 14: 1132-43.
PubMed ID: 1773700 CLOSE
CLOSE
Després JP and Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881-7.
PubMed ID: 17167477 CLOSE
CLOSE
Hartz AJ, Rupley DC, Jr., Kalkhoff RD, et al. Relationship of obesity to diabetes: influence of obesity level and body fat distribution. Prev Med 1983; 12: 351-7.
PubMed ID: 6878197 CLOSE
CLOSE
Feldman R, Sender AJ and Siegelaub AB. Difference in diabetic and nondiabetic fat distribution patterns by skinfold measurements. Diabetes 1969; 18: 478-86.
PubMed ID: 5795030 CLOSE
CLOSE
Lundgren H, Bengtsson C, Blohme G, et al. Adiposity and adipose tissue distribution in relation to incidence of diabetes in women: results from a prospective population study in Gothenburg, Sweden. Int J Obes 1989; 13: 413-23.
PubMed ID: 2793297 CLOSE
CLOSE
Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 1997; 145: 614-9.
PubMed ID: 9098178 CLOSE
CLOSE
Wang Y, Rimm EB, Stampfer MJ, et al. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005; 81: 555-63.
PubMed ID: 15755822 CLOSE
CLOSE
Després JP, Moorjani S, Lupien PJ, et al. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 1990; 10: 497-511.
PubMed ID: 2196040 CLOSE
CLOSE
Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med 2006; 38: 52-63.
PubMed ID: 16448989 CLOSE
CLOSE
Frayn KN, Karpe F, Fielding BA, et al. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord 2003; 27: 875-88.
PubMed ID: 12861227 CLOSE
CLOSE
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21: 697-738.
PubMed ID: 11133069 CLOSE
CLOSE
Fujioka S, Matsuzawa Y, Tokunaga K, et al. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 1987; 36: 54-9.
PubMed ID: 3796297 CLOSE
CLOSE
Pouliot MC, Després JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 1992; 41: 826-34.
PubMed ID: 1612197 CLOSE
CLOSE
Ross R, Aru J, Freeman J, et al. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab 2002; 282: E657-63.
PubMed ID: 11832370 CLOSE
CLOSE
Ross R, Freeman J, Hudson R, et al. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab 2002; 87: 5044-51.
PubMed ID: 12414870 CLOSE
CLOSE
Alberti KG, Zimmet P and Shaw J. The metabolic syndrome–a new worldwide definition. Lancet 2005; 366: 1059-62.
PubMed ID: 16182882 CLOSE
CLOSE
Boyko EJ, Fujimoto WY, Leonetti DL, et al. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 2000; 23: 465-71.
PubMed ID: 10857936 CLOSE
CLOSE
Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020; 16: 177-189.
PubMed ID: 32020062 CLOSE
CLOSE
Neeland IJ, Ross, R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019; 7: 715-25.
PubMed ID: 31301983