Inflammation
Defining CMR - Visceral Adipose Tissue: the Culprit? Complications of Visceral ObesityKey Points
- Adipose tissue is more than an organ that mobilizes energy stored in the form of triglycerides. It is also an endocrine organ that secretes adipokines, which are involved in the atherogenic/diabetogenic metabolic risk profile of abdominal obesity.
- Obesity, especially visceral obesity, is associated with chronic low-grade inflammation.
- This inflammation may lead to insulin resistance and other features of the metabolic syndrome associated with visceral obesity, such as dyslipidemia.
- The inflammatory component of CHD has been emphasized in recent years and may increase risk of acute coronary syndrome. However, hs-CRP may be a marker rather than a cause of metabolic disturbances that increase CVD risk.
Inflammation and Obesity
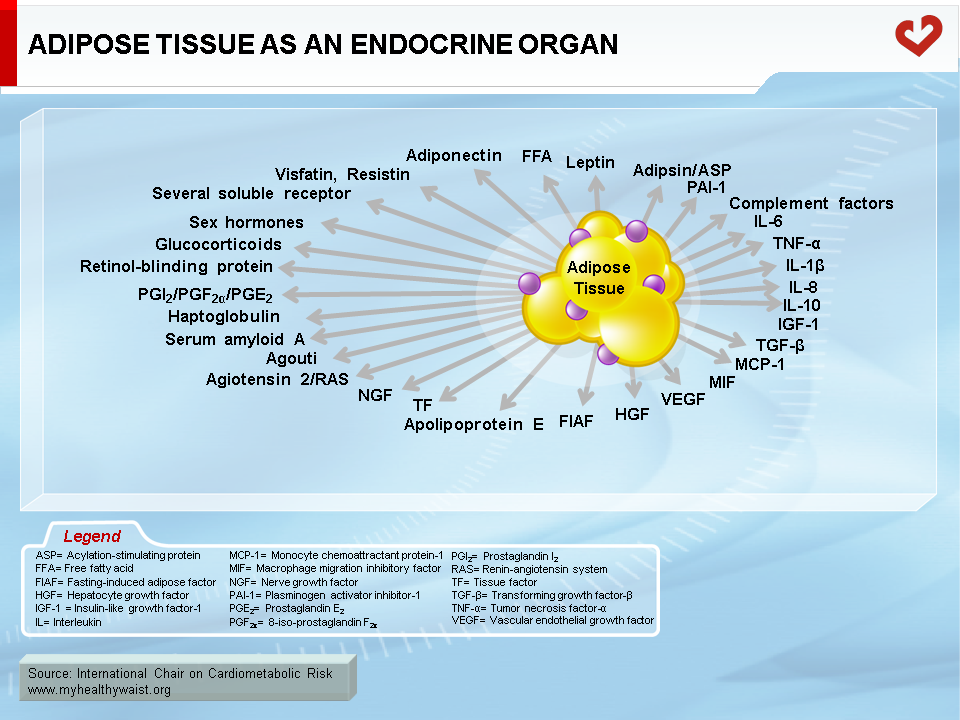
It is now clear that adipose tissue is much more than an organ that mobilizes energy stored in the form of triglycerides. Adipose tissue is now also considered an endocrine, paracrine, and autocrine organ that has a major impact on energy metabolism and related risk of diabetes and cardiovascular disease (CVD) (1). Leptin’s discovery in 1994 was a milestone in that it firmly established the true endocrine function of adipose tissue (2). Adipose tissue is now known to express and secrete a variety of bioactive peptides known as adipokines (Figure 1) that act at both a local (autocrine/paracrine) and systemic (endocrine) level. Among the wide variety of adipokines, adipocytes synthesize and secrete proteins such as classical cytokines (tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6)), growth factors (transforming growth factor-β), and proteins of the alternative complement system (adipsin, acylation-stimulating protein). Adipocytes also secrete proteins that participate in lipid transport (cholesterol ester transfer protein (CETP), retinol-binding protein (RBP)), vascular haemostasis (plasminogen activator inhibitor-1 (PAI-1), regulation of blood pressure (angiotensinogen), and glucose homeostasis (adiponectin). In addition to these efferent signals, adipose tissue expresses numerous receptors that allow it to respond to afferent signals from traditional hormone systems and the central nervous system.
Obesity, abdominal obesity in particular, is known to be associated with chronic low-grade inflammation (3). This view is based on the fact that circulating levels of several inflammation markers, both pro-inflammatory cytokines (TNF-α and IL-6) and acute-phase proteins (high-sensitive C-reactive protein (hs-CRP)), are elevated in obesity. Moreover, several inflammatory cytokines are now recognized to be expressed in and secreted by adipose tissue in obese individuals. The first to be identified was TNF-α (4), and the other key cytokine is IL-6 (5). Both influence lipid and glucose-insulin metabolism (Figure 2).
It has recently been suggested that macrophage infiltration could explain this overexpression of proinflammatory molecules by adipose tissue as obesity develops (6). These inflammatory changes are most pronounced within visceral fat, the fat depot associated with greatest metabolic risk, and seem to precede other features of the metabolic syndrome (7). Accordingly, it has been reported that large amounts of visceral adipose tissue markedly increase plasma hs-CRP concentrations (8). Significant associations between hs-CRP levels and features of the metabolic syndrome, such as an elevated waist circumference, low HDL cholesterol levels, and increased triglyceride concentrations, have also been reported (3,8). Adipose tissue production of IL-6 rises in step with adiposity. Circulating IL-6 concentrations are also associated with obesity (5) and are mostly produced and secreted by visceral adipose tissue (9). Moreover, it has been estimated that adipose tissue contributes about 30% of circulating IL-6. In contrast, previous studies have shown that both TNF-α mRNA content and TNF-α secretion increase in the adipose tissue of obese individuals (10), but there is a lack of depot-related difference in mRNA expression of TNF-α (11,12). It remains unclear whether TNF-α secretion by adipose tissue directly accounts for the elevated serum TNF-α concentration seen in obesity. It has been suggested that TNF-α acts locally on adipose tissue through autocrine/paracrine mechanisms that affect insulin resistance and induce IL-6, whereas IL-6 appears to be released systemically by the adipose tissue and acts more as an endocrine signal that induces hepatic acute-phase response or insulin resistance in the skeletal muscle (13).
Inflammation and Insulin Resistance
Inflammation is increasingly acknowledged as an important component of both CVD (14) and type 2 diabetes (15). Many studies have linked inflammation to insulin resistance (16), which could even predict type 2 diabetes (17,18).
There is evidence to suggest that TNF-α and IL-6 play a role in obesity-linked insulin resistance. These findings are consistent with previous studies that showed increased levels of circulating TNF-α and IL-6 among subjects with insulin resistance (19-23). Although it is unclear how adipose TNF-α and IL-6 expression may cause insulin resistance, they have both been shown to interfere with insulin signalling in adipose tissue (24,25) and the liver (4,26). Rotter et al. (24) reported that, unlike TNF-α, IL-6 did not increase serine phosphorylation of insulin receptor substrate-1 (IRS-1). Instead, similar to TNF-α it suppressed gene transcription of IRS-1 and GLUT-4 (glucose transporters) as well as peroxisome proliferator-activated receptor gamma (PPAR-γ) in adipose tissue. The authors also demonstrated that IL-6 is mainly a chronic modulator of insulin action and that its effects may be tissue-specific (27). TNF-α (28) and IL-6 (29) are also known to promote lipolysis and secretion of free fatty acids (FFA) from adipose tissue into the circulation, which contributes to insulin resistance, increases hepatic glucose production, and reduces hepatic insulin extraction. A third potential mechanism by which TNF-α influences insulin resistance has also recently been identified. TNF-α signalling strongly reduces adiponectin secretion by adipocytes (30), and TNF-α seems to be a crucial mediator of insulin sensitivity. This may explain how the paracrine effects of TNF-α within fat could cause systemic insulin resistance. Another proposed mechanism is the induction by proinflammatory cytokines of suppressor of cytokines signalling (SOCS) proteins, which are known to alter insulin signalling and could therefore play a role in mediating cytokine-dependent insulin resistance in the liver and other insulin-responsive tissues (31).
Furthermore, insulin is known to inhibit the release of cytokines, such as IL-6, that stimulate acute-phase protein gene expression (32,33). If insulin action is impaired, insulin would therefore no longer be able to block IL-6 release, causing a prolonged acute-phase reaction (22).
These findings are consistent with the hypothesis that proinflammatory cytokines secreted by adipose tissue may link obesity to insulin resistance (3,21) (Figure 2).
Inflammation and Dyslipidemia
Cytokine-induced alterations in lipid and lipoprotein metabolism often accompany infection and inflammation. Inflammatory cytokines are elevated and play a pathogenic role in disorders such as diabetes, obesity, the metabolic syndrome, and CVD. Many of these disorders present lipid metabolism abnormalities that are similar to those that occur during infection and inflammation.
The characteristic lipid metabolism disturbances found during inflammation are increased triglyceride concentrations and decreased HDL cholesterol levels. Inflammation and atherogenic dyslipidemia may be linked by the fact that TNF-α and IL-6 stimulate lipolysis, increasing FFA flux to the liver (Figure 2). This increase in FFA induces hepatic triglyceride synthesis (by increasing hepatic de novo fatty acid synthesis or the reesterification of fatty acids derived from peripheral lipolysis) and increases liver VLDL secretion, both of which increase hepatic triglyceride production and secretion causing hypertriglyceridemia (34). TNF-α and IL-6 also suppress lipoprotein lipase synthesis in adipose tissue, which could contribute to the hypertriglyceridemia and low HDL cholesterol concentrations observed in individuals with visceral obesity (35). With respect to low HDL cholesterol, inflammation and cytokines can modify the size, composition, and function of HDL particles in several ways. Inflammation-related changes to HDL particles not only lower HDL cholesterol levels but also alter the anti-oxidant properties of HDL. It is thought that the lowering of HDL cholesterol levels during inflammation is due to a reduction in cholesterol uptake by cells and an increase in their catabolism (36).
Inflammation and CHD
It is now accepted that atherosclerosis has an inflammatory component. Compelling evidence suggests that inflammation plays a central role at every stage of atherogenesis, such as the formation, maturation, and degradation of the atherosclerotic plaque, the initiation of endothelial dysfunction, and the promotion of thrombus formation (37).
The inflammatory component of coronary heart disease (CHD) has been emphasized in recent years, and it has been proposed that it could predict increased risk of acute coronary syndrome by being a marker of atherosclerotic plaque instability (14). For instance, elevated levels of hs-CRP, a marker of a low chronic inflammation state, have been shown to increase risk of myocardial infarction in patients with both stable and unstable CHD (38-40) as well as in healthy individuals (41-44). However, it remains unclear whether hs-CRP is an independent risk factor for CHD. A meta-analysis revealed that hs-CRP concentration was a relatively moderate predictor of CHD risk and that it added only marginally to the predictive value of established CHD risk factors (45). Also, there is no direct evidence to suggest that hs-CRP has a proatherogenic role in vivo (46). Thus, an elevated hs-CRP concentration may therefore only be a marker (albeit a good one) of metabolic disturbances that increase risk of CVD.
Adipose tissue is a complex and highly active metabolic and endocrine organ. The emergence of the concept that obesity is characterized by chronic low-grade inflammation has been an important development in our understanding of obesity. Moreover, the dysregulation of adipokine production and secretion by the adipose tissue in the context of abdominal obesity may be linked to the development of metabolic abnormalities and CVD. In addition, although the clinical relevance of hs-CRP measurements in predicting CHD risk remains unproven, further research into inflammatory response will shed new light on the mechanisms of obesity, insulin resistance, diabetes, and athero-thrombosis.
References
-
Flier JS. The adipocyte: storage depot or node on the energy information superhighway? Cell 1995; 80: 15-8
PubMed ID: 7813011
-
Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425-32.
PubMed ID: 7984236
-
Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999; 19: 972-8.
PubMed ID: 10195925
-
Hotamisligil GS, Shargill NS and Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87-91.
PubMed ID: 7678183
-
Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 1997; 82: 4196-200.
PubMed ID: 9398739
-
Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796-808.
PubMed ID: 14679176
-
Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004; 15: 2792-800.
PubMed ID: 15504932
-
Lemieux I, Pascot A, Prud’homme D, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol 2001; 21: 961-7.
PubMed ID: 11397704
-
Fried SK, Bunkin DA and Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998; 83: 847-50.
PubMed ID: 9506738
-
Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409-15.
PubMed ID: 7738205
-
Montague CT, Prins JB, Sanders L, et al. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes 1998; 47: 1384-91.
PubMed ID: 9726225
-
Fain JN, Bahouth SW and Madan AK. TNFalpha release by the nonfat cells of human adipose tissue. Int J Obes Relat Metab Disord 2004; 28: 616-22.
PubMed ID: 14770194
-
Mohamed-Ali V, Goodrick S, Bulmer K, et al. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol 1999; 277: E971-5.
PubMed ID: 10600783
-
Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999; 340: 115-26.
PubMed ID: 9887164
-
Pickup JC and Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 1998; 41: 1241-8.
PubMed ID: 9794114
-
Festa A, D’Agostino R, Jr., Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000; 102: 42-7.
PubMed ID: 10880413
-
Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 1999; 353: 1649-52.
PubMed ID: 10335783
-
Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286: 327-34.
PubMed ID: 11466099
-
Pickup JC, Chusney GD, Thomas SM, et al. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci 2000; 67: 291-300.
PubMed ID: 10983873
-
Mishima Y, Kuyama A, Tada A, et al. Relationship between serum tumor necrosis factor-alpha and insulin resistance in obese men with type 2 diabetes mellitus. Diabetes Res Clin Pract 2001; 52: 119-23.
PubMed ID: 11311966
-
Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001; 280: E745-51.
PubMed ID: 11287357
-
Fernandez-Real JM, Vayreda M, Richart C, et al. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab 2001; 86: 1154-9.
PubMed ID: 11238501
-
Bastard JP, Maachi M, Van Nhieu JT, et al. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab 2002; 87: 2084-9.
PubMed ID: 11994345
-
Rotter V, Nagaev I and Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 2003; 278: 45777-84.
PubMed ID: 12952969
-
Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord 2003; 27 Suppl 3: S53-5.
PubMed ID: 14704746
-
Senn JJ, Klover PJ, Nowak IA, et al. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002; 51: 3391-9.
PubMed ID: 12453891
-
Rotter Sopasakis V, Larsson BM, Johansson A, et al. Short-term infusion of interleukin-6 does not induce insulin resistance in vivo or impair insulin signalling in rats. Diabetologia 2004; 47: 1879-87.
PubMed ID: 15551046
-
Ruan H and Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev 2003; 14: 447-55.
PubMed ID: 12948526
-
Stouthard JM, Romijn JA, Van der Poll T, et al. Endocrinologic and metabolic effects of interleukin-6 in humans. Am J Physiol 1995; 268: E813-9.
PubMed ID: 7762632
-
Kern PA, Di Gregorio GB, Lu T, et al. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 2003; 52: 1779-85.
PubMed ID: 12829646
-
Senn JJ, Klover PJ, Nowak IA, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 2003; 278: 13740-6.
PubMed ID: 12560330
-
Campos SP and Baumann H. Insulin is a prominent modulator of the cytokine-stimulated expression of acute-phase plasma protein genes. Mol Cell Biol 1992; 12: 1789-97.
PubMed ID: 1372389
-
Andersson CX, Sopasakis VR, Wallerstedt E, et al. Insulin antagonizes interleukin-6 signaling and is anti-inflammatory in 3T3-L1 adipocytes. J Biol Chem 2007; 282: 9430-5.
PubMed ID: 17267401
-
Feingold KR, Doerrler W, Dinarello CA, et al. Stimulation of lipolysis in cultured fat cells by tumor necrosis factor, interleukine-1, and the interferons is blocked by inhibition of prostaglandin synthesis. Endocrinology 1992; 130: 10-6.
PubMed ID: 1370149
-
Kawakami M and Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med 1981; 154: 631-9.
PubMed ID: 7276825
-
Esteve E, Ricart W and Fernandez-Real JM. Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr 2005; 24: 16-31.
PubMed ID: 15681098
-
Morrow DA and Ridker PM. C-reactive protein, inflammation, and coronary risk. Med Clin North Am 2000; 84: 149-61, ix.
PubMed ID: 10685132
-
Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med 1994; 331: 417-24.
PubMed ID: 7880233
-
Thompson SG, Kienast J, Pyke SD, et al. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med 1995; 332: 635-41.
PubMed ID: 7845427
-
Haverkate F, Thompson SG, Pyke SD, et al. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet 1997; 349: 462-6.
PubMed ID: 9040576
-
Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol 1997; 17: 1121-7.
PubMed ID: 9194763
-
Kuller LH, Tracy RP, Shaten J, et al. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol 1996; 144: 537-47.
PubMed ID: 8797513
-
Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336: 973-9.
PubMed ID: 9077376
-
Koenig W, Sund M, Frohlich M, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation 1999; 99: 237-42.
PubMed ID: 9892589
-
Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350: 1387-97.
PubMed ID: 15070788
-
Trion A, de Maat MP, Jukema JW, et al. No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-leiden/human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol 2005; 25: 1635-40.
PubMed ID: 15920036