Pro-thrombotic State
Defining CMR - Visceral Adipose Tissue: the Culprit? Complications of Visceral ObesityKey Points
- Obese, insulin-resistant subjects and type 2 diabetic patients often have a pro-thrombotic state.
- Atherothrombotic complications of the metabolic syndrome are partly due to a dysregulation of hemostasis. This induces a pro-thrombotic state that includes endothelial activation, platelet hyperactivity, hypercoagulability, and hypofibrinolysis.
- Hypofibrinolysis because of elevated PAI-1 levels is a core feature of the metabolic syndrome.
- Weight loss improves all pro-thrombotic factors, indicating that obesity is a modifiable risk factor for thrombosis.
Fat Distribution and Pro-thrombotic State
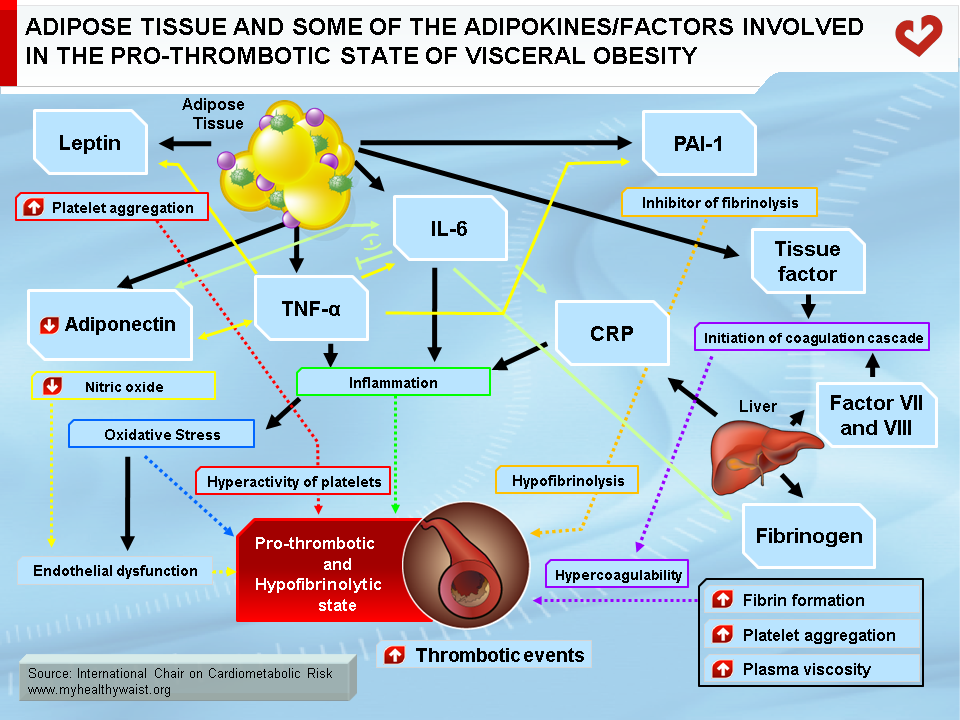
Obesity and overweight pose a major risk for serious chronic diseases, including type 2 diabetes, cardiovascular disease, hypertension, and stroke (1,2). However, it remains to be determined whether obesity is truly an independent risk factor or whether it acts in conjunction with other well-known cardiovascular risk factors such as insulin resistance, dyslipidemia, and a pro-inflammatory and pro-thrombotic state. This cluster of atherogenic metabolic abnormalities is now often referred to as the metabolic syndrome. In addition, obesity is a heterogeneous condition (3). Numerous studies have revealed that the amount of body fat does not necessarily determine morbidity and that body fat distribution is most closely linked to metabolic and vascular diseases (3). Several reports have demonstrated that visceral fat is associated with significant, and largely preventable, morbidity and mortality, including an increased incidence and prevalence of arterial and venous thrombotic events (4). Moreover, atherothrombotic complications in the metabolic syndrome are partly due to a dysregulation of hemostasis, inducing a pro-thrombotic state that encompasses endothelial activation, platelet hyperactivity, hypercoagulability, and hypofibrinolysis (Figure). All of these changes can be found in abdominally obese, insulin-resistant subjects. However, increased plasminogen activator inhibitor-1 (PAI-1) expression with attendant hypofibrinolysis is the main hemostasis disorder linked to insulin resistance and is now considered part of the cluster of abnormalities of the metabolic syndrome (5).
Hypofibrinolysis
Adipose tissue is much more than an organ that mobilizes energy stored in the form of triglycerides. It is also an endocrine organ that secretes adipokines that contribute to the atherogenic/diabetogenic metabolic risk profile of abdominal obesity (6). Among the wide variety of bioactive substances called adipokines, several may play a role in thrombosis. A key adipokine is PAI-1. It inhibits plasminogen activator (tPA), which cleaves plasmin from plasminogen and is therefore the primary physiological inhibitor of fibrinolysis in vivo (7) (Figure). Several tissues produce PAI-1, including the liver, spleen, and adipocytes (8). Elevated PAI-1 concentrations hinder normal clearance of fibrin and promote thrombosis (9), which increases the risk of myocardial infarction and stroke (10). The link between PAI-1 and the metabolic syndrome was first described by Dr. Irene Juhan-Vague’s group in 1986 and is now well established (11).
Circulating PAI-1 levels are elevated in obese subjects with the metabolic syndrome and in patients with type 2 diabetes. The more severe the metabolic syndrome, the higher plasma PAI-1 levels (12). PAI-1 levels are associated with high-risk abdominal obesity as established through waist circumference and insulin resistance (13). Several groups have also reported that increased PAI-1 levels and visceral obesity are closely linked (10,14,15). For example, changes in plasma PAI-1 levels during a weight-loss program have been shown to be related with changes in visceral fat but not in subcutaneous fat (16). In addition, treatment with insulin-sensitizing drugs like metformin or troglitazone decreases plasma PAI-1 levels in subjects with type 2 diabetes and in obese subjects (17). These findings justify the proposal of increased PAI-1 levels (or at least the pro-thrombotic state) as a true component of the metabolic syndrome (5,18).
Platelet Hyperactivity
Leptin is mainly produced and secreted by adipose tissue and acts via the hypothalamus to suppress food intake and increase energy expenditure by modulating glucose and fat metabolism and enhancing thermogenesis (7). Most obese people have high circulating leptin concentrations, which is a signal sent by adipose tissue to limit caloric intake and restore energy balance by reducing appetite and increasing energy expenditure (19). It is therefore generally accepted that overweight and obese individuals are resistant to the effects of leptin (20). The leptin receptor is present in many tissues, including platelets (21). Leptin promotes human platelet aggregation by increasing normal platelet response to the agonists adenosine diphosphate and thrombin (Figure). This has recently been suggested as one mechanism responsible for acute thrombotic events in obesity (22).
Hypercoagulability
Increased fibrinogen concentrations are a consistent plasma abnormality in obesity. Fibrinogen promotes arterial and venous thrombosis by increasing fibrin formation, platelet aggregation, and plasma viscosity, and it promotes atherosclerosis by fostering the proliferation of vascular smooth muscle and endothelial cells (23) (Figure). Fibrinogen levels are now considered to be more closely related to overall adiposity than insulin resistance (24). The plasma levels of other clotting factors, such as tissue factor (8) and factor VII and VIII, are also elevated in obese patients (23). Several studies have shown that weight loss can reverse numerous disturbances in the plasma coagulation cascade (25).
Obesity and Inflammation
Obesity is a chronic, low-grade inflammatory state, as demonstrated by increased levels of the pro-inflammatory cytokines interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) and acute phase proteins such as C-reactive protein (CRP) (26). Weight loss has a positive impact on this pro-inflammatory state (27). In addition to its direct effects, inflammation may cause thrombosis indirectly by inducing oxidative stress and endothelial dysfunction (28). IL-6 may promote thrombosis indirectly by increasing platelet count and aggregation, hepatic synthesis of fibrinogen and CRP, and endothelial adhesion molecule expression, as well as by decreasing adiponectin secretion (29). TNF-α stimulates leptin production and reduces adiponectin secretion by adipose tissue, induces PAI-1 expression in adipose tissue, and promotes endothelial adhesion molecule expression (7) (Figure).
Oxidative Stress
Oxidative stress is where the normal balance between the body’s pro-oxidant and anti-oxidant systems is disturbed in favour of oxidation (30). Oxidative stress from the production of reactive oxygen species (ROS) promotes endothelial dysfunction, platelet aggregation, and thrombus formation (31) (Figure). Many obesity-related metabolic abnormalities, such as hyperglycemia, dyslipidemia, and inflammation, all increase ROS production in the endothelium, which promotes oxidative stress (32).
Endothelial Dysfunction
Endothelial dysfunction is a disturbance in the normal balance between vasoconstrictors and vasodilators, growth promoters and inhibitors, pro- and anti-atherogenic processes, and pro- and anti-coagulant factors (33). Endothelial dysfunction is central to the development and progression of atherosclerosis and enhances the risk of future cardiovascular events (33). Endothelial dysfunction is present in overweight patients, especially those with visceral obesity and insulin resistance, and weight loss can improve endothelial function (27). As described above, low levels of adipocyte-derived circulating adiponectin may cause endothelial damage, possibly by lowering nitric oxide (NO) production while increasing ROS (31). Not only does NO reduce inflammation, platelet aggregation, vascular smooth muscle cell migration and growth, and monocyte and macrophage adhesion, it also encourages vasodilatation (33). Low NO production may therefore help increase platelet activation, arterial thrombosis (Figure), and atherogenesis.
Several new features have been added to the metabolic syndrome because they frequently accompany the syndrome’s traditional features. A pro-thrombotic state has been found in obese, insulin-resistant subjects likely to have abdominal obesity. Moreover, increased PAI-1 levels, the main hemostatic disorder linked to insulin resistance, is now considered part of the cluster of abnormalities of the metabolic syndrome. In addition, intervention studies have shown that weight loss through a hypocaloric diet and exercise or treatment with insulin-sensitizing agents are effective in reducing this procoagulant state. This evidence lends weight to the notion that obesity is a modifiable risk factor for thrombosis.
References
-
Wilson PW, D’Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002; 162: 1867-72.
PubMed ID: 12196085
-
Chan JM, Rimm EB, Colditz GA, et al. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994; 17: 961-9.
PubMed ID: 7988316
-
Abate N and Garg A. Heterogeneity in adipose tissue metabolism: causes, implications and management of regional adiposity. Prog Lipid Res 1995; 34: 53-70.
PubMed ID: 7644553
-
Darvall KA, Sam RC, Silverman SH, et al. Obesity and thrombosis. Eur J Vasc Endovasc Surg 2007; 33: 223-33.
PubMed ID: 17185009
-
Juhan-Vague I, Alessi MC and Vague P. Increased plasma plasminogen activator inhibitor 1 levels. A possible link between insulin resistance and atherothrombosis. Diabetologia 1991; 34: 457-62.
PubMed ID: 1916049
-
Flier JS. The adipocyte: storage depot or node on the energy information superhighway? Cell 1995; 80: 15-8.
PubMed ID: 7813011
-
Kershaw EE and Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548-56.
PubMed ID: 15181022
-
Loskutoff DJ and Samad F. The adipocyte and hemostatic balance in obesity: studies of PAI-1. Arterioscler Thromb Vasc Biol 1998; 18: 1-6.
PubMed ID: 9445248
-
Alessi MC, Peiretti F, Morange P, et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes 1997; 46: 860-7.
PubMed ID: 9133556
-
Shimomura I, Funahashi T, Takahashi M, et al. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med 1996; 2: 800-3.
PubMed ID: 8673927
-
Vague P, Juhan-Vague I, Aillaud MF, et al. Correlation between blood fibrinolytic activity, plasminogen activator inhibitor level, plasma insulin level, and relative body weight in normal and obese subjects. Metabolism 1986; 35: 250-3.
PubMed ID: 3081778
-
Juhan-Vague I, Alessi MC, Mavri A, et al. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost 2003; 1: 1575-9.
PubMed ID: 12871293
-
Sakkinen PA, Wahl P, Cushman M, et al. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol 2000; 152: 897-907.
PubMed ID: 11092431
-
Vague P, Juhan-Vague I, Chabert V, et al. Fat distribution and plasminogen activator inhibitor activity in nondiabetic obese women. Metabolism 1989; 38: 913-5.
PubMed ID: 2505018
-
Cigolini M, Targher G, Bergamo Andreis IA, et al. Visceral fat accumulation and its relation to plasma hemostatic factors in healthy men. Arterioscler Thromb Vasc Biol 1996; 16: 368-74.
PubMed ID: 8630661
-
Janand-Delenne B, Chagnaud C, Raccah D, et al. Visceral fat as a main determinant of plasminogen activator inhibitor 1 level in women. Int J Obes Relat Metab Disord 1998; 22: 312-7.
PubMed ID: 9578235
-
Kruszynska YT, Yu JG, Olefsky JM, et al. Effects of troglitazone on blood concentrations of plasminogen activator inhibitor 1 in patients with type 2 diabetes and in lean and obese normal subjects. Diabetes 2000; 49: 633-9.
PubMed ID: 10871202
-
Mertens I, Verrijken A, Michiels JJ, et al. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes (Lond) 2006; 30: 1308-14.
PubMed ID: 16389265
-
Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997; 387: 903-8.
PubMed ID: 9202122
-
Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 2002; 13: 51-9.
PubMed ID: 11790963
-
Nakata M, Yada T, Soejima N, et al. Leptin promotes aggregation of human platelets via the long form of its receptor. Diabetes 1999; 48: 426-9.
PubMed ID: 10334326
-
Konstantinides S, Schafer K, Koschnick S, et al. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest 2001; 108: 1533-40.
PubMed ID: 11714745
-
Reiner AP, Siscovick DS and Rosendaal FR. Hemostatic risk factors and arterial thrombotic disease. Thromb Haemost 2001; 85: 584-95.
PubMed ID: 11341490
-
Juhan-Vague I, Morange P, Renucci JF, et al. Fibrinogen, obesity and insulin resistance. Blood Coagul Fibrinolysis 1999; 10 Suppl 1: S25-8.
PubMed ID: 10070814
-
Folsom AR, Qamhieh HT, Wing RR, et al. Impact of weight loss on plasminogen activator inhibitor (PAI-1), factor VII, and other hemostatic factors in moderately overweight adults. Arterioscler Thromb 1993; 13: 162-9.
PubMed ID: 8427853
-
Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999; 19: 972-8.
PubMed ID: 10195925
-
Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002; 105: 804-9.
PubMed ID: 11854119
-
Sonnenberg GE, Krakower GR and Kissebah AH. A novel pathway to the manifestations of metabolic syndrome. Obes Res 2004; 12: 180-6.
PubMed ID: 14981209
-
Woods A, Brull DJ, Humphries SE, et al. Genetics of inflammation and risk of coronary artery disease: the central role of interleukin-6. Eur Heart J 2000; 21: 1574-83.
PubMed ID: 10988009
-
Block G, Dietrich M, Norkus EP, et al. Factors associated with oxidative stress in human populations. Am J Epidemiol 2002; 156: 274-85.
PubMed ID: 12142263
-
Loscalzo J. Oxidant stress: a key determinant of atherothrombosis. Biochem Soc Trans 2003; 31: 1059-61.
PubMed ID: 14505479
-
Davi G, Guagnano MT, Ciabattoni G, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA 2002; 288: 2008-14.
PubMed ID: 12387653
-
Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res 2003; 11: 1278-89.
PubMed ID: 14627747