The Concept of Energy Balance
Defining CMR - The Obesity and Type 2 Diabetes EpidemicsKey Points
- Energy intake should be seen as a behaviour which is often influenced by social, environmental, and psychological factors.
- The interaction between peripheral organs and the hypothalamus has an impact on hunger, motivation to eat, satiety, and energy balance.
- The macronutrient composition of the diet also influences energy intake and energy expenditure.
- Obesity and related comorbidities occur when energy intake exceeds energy expenditure over time.
- In sedentary individuals, resting metabolic rate accounts for a large part of total energy expenditure.
- Physical activity increases energy expenditure by increasing resting metabolic rate, enhancing sensitivity to specific hormones, and decreasing post-exercise energy intake.
- Physical activity has the greatest impact on total daily energy expenditure. However, a high level of physical activity is required for this to occur.
- Exercise benefits cardiovascular health and risk factors beyond what can be explained by increased energy expenditure.
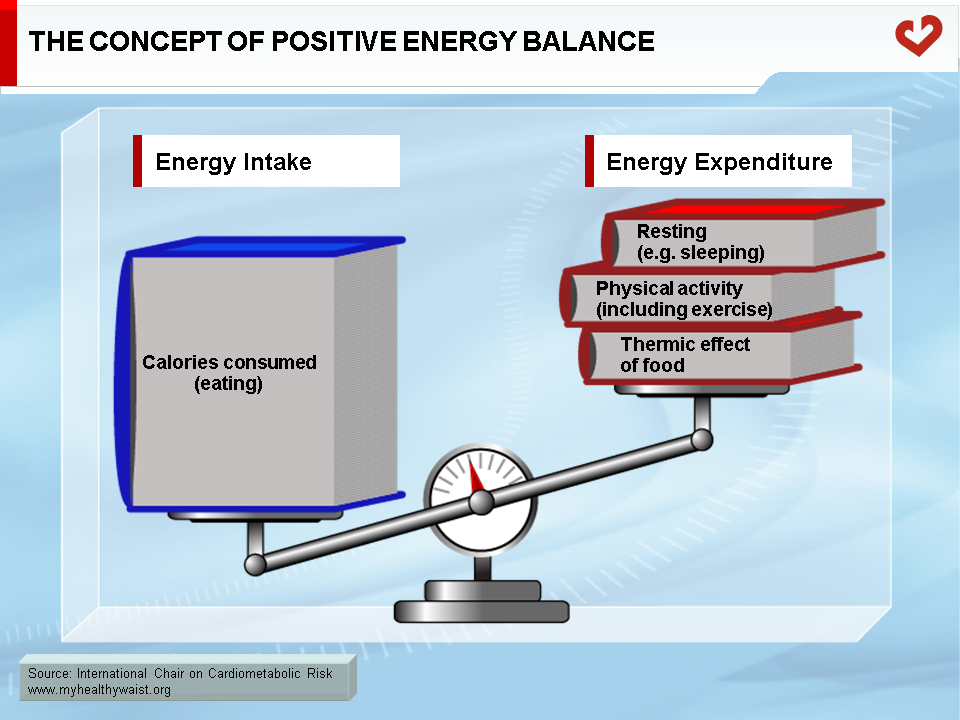
The Concept of Energy Balance
The growing obesity epidemic is a multifaceted public health problem caused in part by the mechanization and computerization of human labour over the last century. Not only are we now less active at work, we also lead a sedentary lifestyle in which food intake often exceeds energy expenditure. This can lead to overweight/obesity over an extended period of time (Figure 1). This major behavioural change has had serious repercussions on the body’s energy storage systems. For instance, we are no longer at risk of negative energy balance, which was common in our hunter-gatherer days. Instead, we face a new problem that coincided with the start of industrialization: positive energy balance. Though we may live in the 21st century, humans carry the genes of their ancestors who were naturally selected to store energy for long periods of limited food availability and even starvation. This genetic background protects humans against weight loss and not weight gain, which makes it very difficult for overweight individuals to lose weight. However, the obesity epidemic cannot be explained by changes in our genes, since this phenomenon has only been around for a few decades. Rather, it is our sedentary, affluent societies and individual genetic susceptibility that work in conjunction to affect energy balance and body weight over time. Thus, our ability to store excess energy is interacting with our environment to send obesity rates soaring around the world. A positive energy balance leading to excess fat storage can be caused by various combinations of factors: excess food intake, decreased energy expenditure, or a combination of both, the latter often being observed in obese patients.
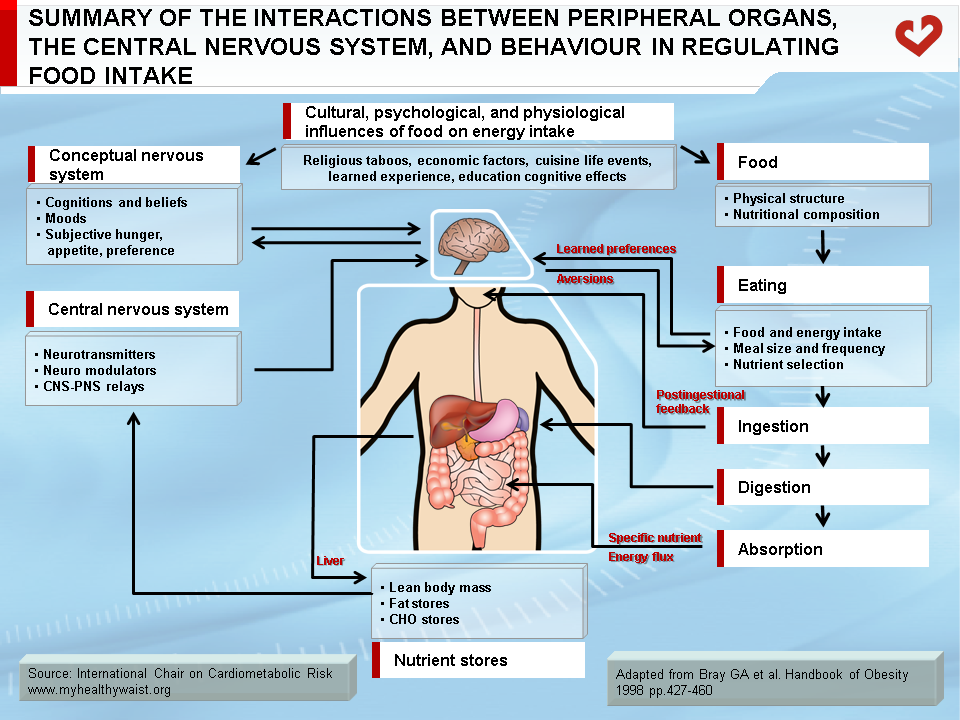
Determinants of Energy Intake
Food intake is controlled by a number of biological and external factors/stimuli. External factors that regulate appetite (and food intake) include cultural and psychological influences such as economic background, education, learned experiences, cognitive effects, portion size, and, especially, food palatability (Figure 2) [1]. Given that a variety of outside influences affect one’s eating habits, eating (and not eating) can be seen as a kind of behaviour in which individual and environmental factors influence the amount of energy ingested. Accordingly, hectic food patterns are known to be a significant cause of obesity in individuals with eating disorders [2]. There are also many metabolic and peripheral pathways that are part of the human appetite and satiety system. This very complex system governs energy intake and expenditure and is responsible for feeding and satiety. Disturbances to this system can affect energy balance and cause weight gain (in most cases) or weight loss. Many sites are involved in the appetite system, and the brain (especially the hypothalamic region) is one of the centres where information (signals) from tissues is analyzed and “orders” (other signals) are sent to the system controlling appetite and eating. The gastro-intestinal (GI) tract, liver, pancreas, and adipose tissue therefore engage in a cross-talk between peripheral organs and the central nervous system (CNS). In response to peripheral signals, the CNS secretes numerous hypothalamic peptides (neuropeptides) that play a role in food intake and energy balance [3].
Neuropeptides and Appetite Regulation
The hypothalamus is frequently referred to as the “feeding” or “satiety” centre. The main regions of the hypothalamus involved in energy intake are the arcuate and the paraventricular nucleus. Both regions secrete orexigenic and anorexigenic peptides.
The main role of orexigenic neuropeptides is to restore body fat stores (i.e., restore energy balance) when energy deficits occur. Neuropeptide Y (NPY) is one of the most abundant orexigenic peptides. It is mostly secreted by neurons of the arcuate nucleus and reaches other hypothalamic targets, such as the paraventricular nucleus and the dorsomedial hypothalamic nucleus, prior to feeding [4]. NPY is present at very high concentrations during starvation, but overeating does not raise hypothalamic NPY levels [5]. Similar to NPY, melanin-concentrating hormone (MCH), a peptide produced in the lateral hypothalamus, uses G-coupled receptors to modulate food intake. MCH receptors are found in the hippocampus, amygdala, and cerebral cortex. Though the role of MCH is not fully understood, it has been shown in rats that acute intracerebrovascular injections of MCH can induce intake of high-calorie, energy-dense food, suggesting that it plays a role in energy balance [6]. Ghrelin is an orexigenic peptide produced by the gut, mostly by the cells of the human gastric mucosa. Circulating ghrelin levels are particularly high before feeding and are acutely downregulated by refeeding [7].
Among anorexigenic peptides, leptin plays a crucial role in energy balance. It is mainly secreted by adipose tissue and is a positive correlate of body fat mass in humans [8]. One of the pivotal roles of leptin is to downregulate orexigenic peptides such as NPY and MCH and the signalling lipid 2-arachidonoylglycerol (2-AG) [9]. Leptin receptors are found in the hypothalamus (in the arcuate and paraventricular nuclei and the dorsomedial hypothalamic nucleus), and starvation suppresses leptin levels, which can be reversed by refeeding [10]. Leptin is known to interact with another peripheral anorexigenic peptide, cholescystokinin (CCK), to downregulate short-term food intake [11]. The intestine quickly secretes CCK during the ingestion of a meal, and CCK is involved in reward behaviour, memory, and, especially, satiety [12]. In the periphery, CCK stimulates the endocrine function of the pancreas and encourages intestinal motility.
Within the hypothalamus, there are a number of neuropeptides involved in satiety. The cocaine and amphetamine regulated transcript (CART) is located in the hypothalamus, specifically the paraventricular nucleus, dorsomedial nucleus, and arcuate [3]. Fasting is known to reduce hypothalamic CART mRNA expression, whereas other anorexigenic peptides, such as leptin, raise CART concentrations to encourage satiety [13]. Glucagon-like peptide 1 (GLP-1) acts in the hypothalamic midline nuclei and inhibits desire to eat and food intake. This effect might be due to the inhibition of NPY by GLP-1 or the interaction between GLP-1 and leptin in reducing food intake and body weight, as demonstrated in rats by Goldstone et al. [14].
Among other mediators of neuropeptide release, endocannabinoids have received a great deal of attention with the characterization of the endocannabinoid system and documentation of its role in energy balance. The two major endocannabinoids known to affect energy balance are 2-AG and anandamide. Endocannabinoids bind to and activate cannabinoid-receptor-1 (CB1), which is located in the brain and in the periphery (adipose tissue, liver, GI tract, skeletal muscle, and pancreas). In the brain, CB1 receptors are found in the limbic system, which plays a role in hedonic evaluation of food. They are also found in the hypothalamus and serve to enhance food intake. It has been shown that men with abdominal (especially visceral) obesity have elevated plasma 2-AG levels, suggesting that endocannabinoids may not only be involved in energy balance, but also in regulating body fat distribution and ectopic fat deposition [15,16]. Antagonism of CB1 receptors fosters weight loss, reduces abdominal fat, and improves the cardiometabolic risk profile of men and women with dyslipidemia [17].
Diet Composition
The chemical composition of food and its relative macronutrient content play a critical role in regulating food intake and satiety. For instance, unlike carbohydrates and dietary fat, proteins are thought to reduce spontaneous food intake by promoting satiety. The amino acid composition of ingested proteins may also be an important regulator of satiety. Fat and alcohol consumption seems to increase energy intake whereas carbohydrate ingestion does not, although there is evidence to suggest that complex carbohydrates (with a low-glycemic index) may induce satiety similar to proteins [18].
Carbohydrates comprise 45 to 60% of a standard diet and are thought to be the central macronutrient regulating energy balance. Carbohydrates vary widely with respect to chemical composition, absorption, and palatability. Dietary fibres and starches are not absorbed quickly and therefore induce satiety faster than carbohydrates with a high-glycemic index such as sucrose, fructose, and sweeteners. The latter three are absorbed quickly and released in the circulation, thereby playing a critical role in food intake, especially in the intake of high-fat, energy-dense foods [19]. High-fat foods are generally quite palatable, and when humans are allowed to eat high-fat foods without restriction, they often eat more of these foods than when asked to eat low-fat foods under the same conditions in a controlled environment. Because of factors such as palatability and energy density, a high-fat diet may therefore promote a positive energy balance and weight gain [20]. Provided that fat is not replaced by refined sugar, low-fat diets can induce a negative energy balance and are recommended by healthcare professionals to treat overweight and obesity [21,22].
As with carbohydrates, the chemical composition of lipids is another factor that can affect energy intake. It has been shown that substituting medium-chain triglycerides for long-chain triglycerides can promote weight loss in men when energy intake is constant [23]. More importantly, polyunsaturated fats have been shown to decrease the activity of certain lipogenic enzymes and increase fatty acid oxidation in mice [24]. It has also been shown that polyunsaturated fatty acids, unlike saturated fatty acids, promote insulin sensitivity and prevent adipocyte enlargement, which is a marker of fat accumulation [25].
In summary, the factors (both environmental and biological) that influence energy intake are quite heterogeneous and may vary considerably among individuals. In order to stem the obesity epidemic and lessen its growing burden, additional research is needed to better understand and integrate determinants of energy intake, from social and environmental factors to biological and neuroendocrine factors.
An Introduction to Energy Expenditure
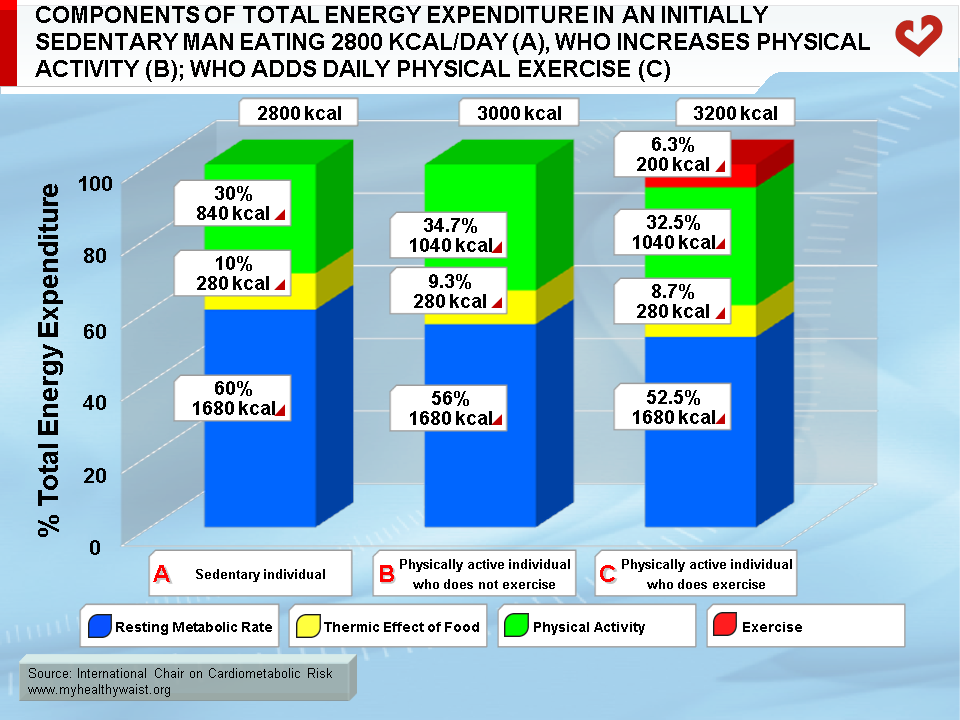
A key component of energy expenditure is resting metabolic rate (RMR). RMR is defined as the energy expended when an individual is lying and resting after sleep. RMR is linked to body size and lean body mass especially. Race and diabetes also affect RMR [26]. In sedentary individuals, RMR is the most important component of daily energy expenditure, accounting for up to 60% of total daily energy expenditure. This percentage is lower in physically active individuals or highly trained athletes [27]. Figure 3 depicts the components of energy expenditure in sedentary individuals, physically active individuals who do not exercise, and physically active individuals who exercise regularly.
The human body strives to maintain intracellular ATP levels (the energy currency) in order to preserve energy homeostasis. To do so, it oxidizes metabolic fuels originating from the diet. Equal amounts of glucose, lipids, and proteins do not generate the same amount of ATP and therefore do not have the same effect on postprandial energy expenditure. This suggests that diet composition can affect not only energy intake but also postprandial energy expenditure. Hurni et al. [28] reported in a group of men that a carbohydrate-rich diet, as opposed to a mixed diet, produced significant increases in RMR and overall energy expenditure, lending weight to the notion that high-fat diets are more likely to cause a positive energy balance. However, as the postprandial increase in energy expenditure cannot be greater than 10% of ingested calories, the difference in energy expenditure resulting from variations in macronutrient intake could not explain large individual differences in daily energy expenditure [27]. Energy expenditure always increases after a meal because of obligatory energy expenditure (digestion, absorption, metabolism and storage of macronutrients) and what has been described as facultative thermogenesis [29]. Facultative energy expenditure includes carbohydrate induction of catecholamine-mediated energy expenditure and activation of the sympathetic nervous system, which enhances lipid oxidation. Along with the thermic effect of food, facultative energy expenditure is part of overall diet-induced thermogenesis. Once considered an interesting theory, the hypothesis that defective facultative thermogenesis may contribute to obesity is no longer considered a significant causal factor because of its rather small effect on total daily energy expenditure.
Physical Activity and Energy Expenditure
Regular physical activity is an excellent way to maintain a healthy body weight and a low-risk metabolic profile. There is a wealth of data proving that physical activity generates cardiovascular and metabolic benefits beyond what can be explained by related energy expenditure.
Regular physical activity can increase resting metabolic rate (RMR), which increases total daily energy expenditure. Sjodin et al. [30] measured the RMR of 8 athletes and compared it to sedentary individuals matched for sex and fat-free mass. They found that for any given amount of fat-free mass, the RMR of athletes was significantly higher than that of sedentary controls. Although the subject is still open to debate, it is generally believed that regular exercise/physical activity can help maintain energy expenditure by having a positive effect on lean body mass (mostly skeletal muscle) [31]. Physical activity also regulates key enzymes involved in energy metabolism. For example, it has been shown that exercise decreases β-adrenoreceptors in the heart while increasing these receptors in skeletal muscle [32,33]. Physical activity also tends to increase post-exercise β-adrenergic stimulation, thereby increasing energy expenditure by boosting lipid oxidation [34]. Moreover, by improving insulin sensitivity, exercise also lowers plasma insulin and glucose levels [35]. Lipoprotein lipase (LPL) is a key enzyme involved in the lipoprotein-lipid metabolism that regulates fat storage in adipose tissue [36]. Exercise reduces LPL activity in adipose tissue but activates LPL activity in skeletal muscle, helping it oxidize more fuel [37]. Exercise also has an impact on LPL response to insulin. For instance, trained athletes are known to have heightened adipose tissue LPL response, which allows them to efficiently replenish adipose tissue lipid stores between exercise bouts [38].
Imbeault et al. [39] quantified the post-exercise energy intake of a group of men submitted to two exercise sessions with the same energy cost. One session was shorter and more intense while the other was longer and less intense. When energy intake relative to expenditure was analyzed by subtracting the surplus of energy expended during exercise from total energy intake, high-intensity exercise led to lower post-exercise energy intake compared to low-intensity exercise sessions. This suggests that high-intensity exercise may lower post-exercise compensation in energy intake.
In summary, the general public should be encouraged to take part in physical activity and exercise whenever possible. Current recommendations include performing at least 30 minutes of moderate-intensity exercise, such as brisk walking, on most days of the week [40]. Regular physical activity can increase daily energy expenditure and create an energy deficit. This deficit can alter energy metabolism and subsequently reduce atherogenic and diabetogenic visceral adipose tissue.
References
-
Blundell JE and Finlayson G. Is susceptibility to weight gain characterized by homeostatic or hedonic risk factors for overconsumption? Physiol Behav 2004; 82: 21-5.
PubMed ID: 15234585
-
Ma Y, Bertone ER, Stanek EJ, 3rd, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 2003; 158: 85-92.
PubMed ID: 12835290
-
Arora S and Anubhuti. Role of neuropeptides in appetite regulation and obesity–a review. Neuropeptides 2006; 40: 375-401.
PubMed ID: 16935329
-
Edwards CM, Abusnana S, Sunter D, et al. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol 1999; 160: R7-12.
PubMed ID: 10077743
-
de Rijke CE, Hillebrand JJ, Verhagen LA, et al. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. J Mol Endocrinol 2005; 35: 381-90.
PubMed ID: 16216917
-
Duncan EA, Proulx K and Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res 2005; 29: 958-64.
PubMed ID: 15976521
-
Schmidt PT, Degerblad M, Lindstrom E, et al. Circulating ghrelin levels after food intake during different phases of the migrating motor complex in man. Eur J Clin Invest 2006; 36: 503-8.
PubMed ID: 16796608
-
Weigle DS, Duell PB, Connor WE, et al. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab 1997; 82: 561-5.
PubMed ID: 9024254
-
Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001; 410: 822-5.
PubMed ID: 11298451
-
Kmiec Z, Pokrywka L, Kotlarz G, et al. Effects of fasting and refeeding on serum leptin, adiponectin and free fatty acid concentrations in young and old male rats. Gerontology 2005; 51: 357-62.
PubMed ID: 16299415
-
Barrachina MD, Martinez V, Wang L, et al. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A 1997; 94: 10455-60.
PubMed ID: 9294232
-
Duncan EA, Davita G and Woods SC. Changes in the satiating effect of cholecystokinin over repeated trials. Physiol Behav 2005; 85: 387-93.
PubMed ID: 15978640
-
Li HY, Hwang HW and Hu YH. Functional characterizations of cocaine- and amphetamine-regulated transcript mRNA expression in rat hypothalamus. Neurosci Lett 2002; 323: 203-6.
PubMed ID: 11959420
-
Goldstone AP, Mercer JG, Gunn I, et al. Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents. FEBS Lett 1997; 415: 134-8.
PubMed ID: 9350983
-
Blüher M, Engeli S, Kloting N, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006; 55: 3053-60.
PubMed ID: 17065342
-
Côté M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007; 31: 692-9.
PubMed ID: 17224929
-
Després JP, Golay A and Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005; 353: 2121-34.
PubMed ID: 16291982
-
Stubbs J, Ferres S and Horgan G. Energy density of foods: effects on energy intake. Crit Rev Food Sci Nutr 2000; 40: 481-515.
PubMed ID: 11186237
-
Galgani J, Aguirre C and Diaz E. Acute effect of meal glycemic index and glycemic load on blood glucose and insulin responses in humans. Nutr J 2006; 5: 22.
PubMed ID: 16953881
-
Stubbs RJ, Harbron CG, Murgatroyd PR, et al. Covert manipulation of dietary fat and energy density: effect on substrate flux and food intake in men eating ad libitum. Am J Clin Nutr 1995; 62: 316-29.
PubMed ID: 7625338
-
Lindstrom J, Peltonen M, Eriksson JG, et al. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia 2006; 49: 912-20.
PubMed ID: 16541277
-
Astrup A, Grunwald GK, Melanson EL, et al. The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int J Obes Relat Metab Disord 2000; 24: 1545-52.
PubMed ID: 11126204
-
Seaton TB, Welle SL, Warenko MK, et al. Thermic effect of medium-chain and long-chain triglycerides in man. Am J Clin Nutr 1986; 44: 630-4.
PubMed ID: 3532757
-
Javadi M, Geelen MJ, Lemmens AG, et al. The influence of dietary linoleic and alpha-linolenic acid on body composition and the activities of key enzymes of hepatic lipogenesis and fatty acid oxidation in mice. J Anim Physiol Anim Nutr (Berl) 2007; 91: 11-8.
PubMed ID: 17217386
-
Huber J, Loffler M, Bilban M, et al. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes (Lond) 2007; 31: 1004-13.
PubMed ID: 17130847
-
Martin K, Wallace P, Rust PF, et al. Estimation of resting energy expenditure considering effects of race and diabetes status. Diabetes Care 2004; 27: 1405-11.
PubMed ID: 15161796
-
Levine JA. Non-exercise activity thermogenesis. Proc Nutr Soc 2003; 62: 667-79.
PubMed ID: 14692603
-
Hurni M, Burnand B, Pittet P, et al. Metabolic effects of a mixed and a high-carbohydrate low-fat diet in man, measured over 24 h in a respiration chamber. Br J Nutr 1982; 47: 33-43.
PubMed ID: 7037049
-
Himms-Hagen J. Role of thermogenesis in the regulation of energy balance in relation to obesity. Can J Physiol Pharmacol 1989; 67: 394-401.
PubMed ID: 2667732
-
Sjodin AM, Forslund AH, Westerterp KR, et al. The influence of physical activity on BMR. Med Sci Sports Exerc 1996; 28: 85-91.
PubMed ID: 8775359
-
Speakman JR and Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc 2003; 62: 621-34.
PubMed ID: 14692598
-
Maki T, Kontula K and Harkonen M. The beta-adrenergic system in man: physiological and pathophysiological response. Regulation of receptor density and functioning. Scand J Clin Lab Invest Suppl 1990; 201: 25-43.
PubMed ID: 1978755
-
Werle EO, Strobel G and Weicker H. Decrease in rat cardiac beta 1- and beta 2-adrenoceptors by training and endurance exercise. Life Sci 1990; 46: 9-17.
PubMed ID: 2153886
-
Yoshioka M, Doucet E, St-Pierre S, et al. Impact of high-intensity exercise on energy expenditure, lipid oxidation and body fatness. Int J Obes Relat Metab Disord 2001; 25: 332-9.
PubMed ID: 11319629
-
Björntorp P, De Jounge K, Sjöström L, et al. The effect of physical training on insulin production in obesity. Metabolism 1970; 19: 631-8.
PubMed ID: 5429278
-
Eckel RH. Lipoprotein lipase: a multifonctional enzyme relevant to common metabolic diseases. N Engl J Med 1989; 320: 1060-8.
PubMed ID: 2648155
-
Ladu MJ, Kapsas H and Palmer WK. Regulation of lipoprotein lipase in muscle and adipose tissue during exercise. J Appl Physiol 1991; 71: 404-9.
PubMed ID: 1718935
-
Mauriège P, Prud’homme D, Marcotte M, et al. Regional differences in adipose tissue metabolism between sedentary and endurance-trained women. Am J Physiol 1997; 273: E497-506.
PubMed ID: 9316438
-
Imbeault P, Saint-Pierre S, Alméras N, et al. Acute effects of exercise on energy intake and feeding behaviour. Br J Nutr 1997; 77: 511-21.
PubMed ID: 9155502
-
Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003; 107: 3109-16.
PubMed ID: 12821592
 CLOSE
CLOSE
Blundell JE and Finlayson G. Is susceptibility to weight gain characterized by homeostatic or hedonic risk factors for overconsumption? Physiol Behav 2004; 82: 21-5.
PubMed ID: 15234585 CLOSE
CLOSE
Ma Y, Bertone ER, Stanek EJ, 3rd, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 2003; 158: 85-92.
PubMed ID: 12835290 CLOSE
CLOSE
Arora S and Anubhuti. Role of neuropeptides in appetite regulation and obesity–a review. Neuropeptides 2006; 40: 375-401.
PubMed ID: 16935329 CLOSE
CLOSE
Edwards CM, Abusnana S, Sunter D, et al. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol 1999; 160: R7-12.
PubMed ID: 10077743 CLOSE
CLOSE
de Rijke CE, Hillebrand JJ, Verhagen LA, et al. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. J Mol Endocrinol 2005; 35: 381-90.
PubMed ID: 16216917 CLOSE
CLOSE
Duncan EA, Proulx K and Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res 2005; 29: 958-64.
PubMed ID: 15976521 CLOSE
CLOSE
Schmidt PT, Degerblad M, Lindstrom E, et al. Circulating ghrelin levels after food intake during different phases of the migrating motor complex in man. Eur J Clin Invest 2006; 36: 503-8.
PubMed ID: 16796608 CLOSE
CLOSE
Weigle DS, Duell PB, Connor WE, et al. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab 1997; 82: 561-5.
PubMed ID: 9024254 CLOSE
CLOSE
Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001; 410: 822-5.
PubMed ID: 11298451 CLOSE
CLOSE
Kmiec Z, Pokrywka L, Kotlarz G, et al. Effects of fasting and refeeding on serum leptin, adiponectin and free fatty acid concentrations in young and old male rats. Gerontology 2005; 51: 357-62.
PubMed ID: 16299415 CLOSE
CLOSE
Barrachina MD, Martinez V, Wang L, et al. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A 1997; 94: 10455-60.
PubMed ID: 9294232 CLOSE
CLOSE
Duncan EA, Davita G and Woods SC. Changes in the satiating effect of cholecystokinin over repeated trials. Physiol Behav 2005; 85: 387-93.
PubMed ID: 15978640 CLOSE
CLOSE
Li HY, Hwang HW and Hu YH. Functional characterizations of cocaine- and amphetamine-regulated transcript mRNA expression in rat hypothalamus. Neurosci Lett 2002; 323: 203-6.
PubMed ID: 11959420 CLOSE
CLOSE
Goldstone AP, Mercer JG, Gunn I, et al. Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents. FEBS Lett 1997; 415: 134-8.
PubMed ID: 9350983 CLOSE
CLOSE
Blüher M, Engeli S, Kloting N, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006; 55: 3053-60.
PubMed ID: 17065342 CLOSE
CLOSE
Côté M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007; 31: 692-9.
PubMed ID: 17224929 CLOSE
CLOSE
Després JP, Golay A and Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005; 353: 2121-34.
PubMed ID: 16291982 CLOSE
CLOSE
Stubbs J, Ferres S and Horgan G. Energy density of foods: effects on energy intake. Crit Rev Food Sci Nutr 2000; 40: 481-515.
PubMed ID: 11186237 CLOSE
CLOSE
Galgani J, Aguirre C and Diaz E. Acute effect of meal glycemic index and glycemic load on blood glucose and insulin responses in humans. Nutr J 2006; 5: 22.
PubMed ID: 16953881 CLOSE
CLOSE
Stubbs RJ, Harbron CG, Murgatroyd PR, et al. Covert manipulation of dietary fat and energy density: effect on substrate flux and food intake in men eating ad libitum. Am J Clin Nutr 1995; 62: 316-29.
PubMed ID: 7625338 CLOSE
CLOSE
Lindstrom J, Peltonen M, Eriksson JG, et al. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia 2006; 49: 912-20.
PubMed ID: 16541277 CLOSE
CLOSE
Astrup A, Grunwald GK, Melanson EL, et al. The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int J Obes Relat Metab Disord 2000; 24: 1545-52.
PubMed ID: 11126204 CLOSE
CLOSE
Seaton TB, Welle SL, Warenko MK, et al. Thermic effect of medium-chain and long-chain triglycerides in man. Am J Clin Nutr 1986; 44: 630-4.
PubMed ID: 3532757 CLOSE
CLOSE
Javadi M, Geelen MJ, Lemmens AG, et al. The influence of dietary linoleic and alpha-linolenic acid on body composition and the activities of key enzymes of hepatic lipogenesis and fatty acid oxidation in mice. J Anim Physiol Anim Nutr (Berl) 2007; 91: 11-8.
PubMed ID: 17217386 CLOSE
CLOSE
Huber J, Loffler M, Bilban M, et al. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes (Lond) 2007; 31: 1004-13.
PubMed ID: 17130847 CLOSE
CLOSE
Martin K, Wallace P, Rust PF, et al. Estimation of resting energy expenditure considering effects of race and diabetes status. Diabetes Care 2004; 27: 1405-11.
PubMed ID: 15161796 CLOSE
CLOSE
Levine JA. Non-exercise activity thermogenesis. Proc Nutr Soc 2003; 62: 667-79.
PubMed ID: 14692603 CLOSE
CLOSE
Hurni M, Burnand B, Pittet P, et al. Metabolic effects of a mixed and a high-carbohydrate low-fat diet in man, measured over 24 h in a respiration chamber. Br J Nutr 1982; 47: 33-43.
PubMed ID: 7037049 CLOSE
CLOSE
Himms-Hagen J. Role of thermogenesis in the regulation of energy balance in relation to obesity. Can J Physiol Pharmacol 1989; 67: 394-401.
PubMed ID: 2667732 CLOSE
CLOSE
Sjodin AM, Forslund AH, Westerterp KR, et al. The influence of physical activity on BMR. Med Sci Sports Exerc 1996; 28: 85-91.
PubMed ID: 8775359 CLOSE
CLOSE
Speakman JR and Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc 2003; 62: 621-34.
PubMed ID: 14692598 CLOSE
CLOSE
Maki T, Kontula K and Harkonen M. The beta-adrenergic system in man: physiological and pathophysiological response. Regulation of receptor density and functioning. Scand J Clin Lab Invest Suppl 1990; 201: 25-43.
PubMed ID: 1978755 CLOSE
CLOSE
Werle EO, Strobel G and Weicker H. Decrease in rat cardiac beta 1- and beta 2-adrenoceptors by training and endurance exercise. Life Sci 1990; 46: 9-17.
PubMed ID: 2153886 CLOSE
CLOSE
Yoshioka M, Doucet E, St-Pierre S, et al. Impact of high-intensity exercise on energy expenditure, lipid oxidation and body fatness. Int J Obes Relat Metab Disord 2001; 25: 332-9.
PubMed ID: 11319629 CLOSE
CLOSE
Björntorp P, De Jounge K, Sjöström L, et al. The effect of physical training on insulin production in obesity. Metabolism 1970; 19: 631-8.
PubMed ID: 5429278 CLOSE
CLOSE
Eckel RH. Lipoprotein lipase: a multifonctional enzyme relevant to common metabolic diseases. N Engl J Med 1989; 320: 1060-8.
PubMed ID: 2648155 CLOSE
CLOSE
Ladu MJ, Kapsas H and Palmer WK. Regulation of lipoprotein lipase in muscle and adipose tissue during exercise. J Appl Physiol 1991; 71: 404-9.
PubMed ID: 1718935 CLOSE
CLOSE
Mauriège P, Prud’homme D, Marcotte M, et al. Regional differences in adipose tissue metabolism between sedentary and endurance-trained women. Am J Physiol 1997; 273: E497-506.
PubMed ID: 9316438 CLOSE
CLOSE
Imbeault P, Saint-Pierre S, Alméras N, et al. Acute effects of exercise on energy intake and feeding behaviour. Br J Nutr 1997; 77: 511-21.
PubMed ID: 9155502 CLOSE
CLOSE
Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003; 107: 3109-16.
PubMed ID: 12821592