Interleukin-6 (IL-6) is a highly proinflammatory molecule in many cells, including adipose and liver cells. It is widely produced, but the adipose tissue accounts for a substantial part (around 30%) [1]. The release of IL-6 is related to adipose cell size and, thus, increased in obesity [2].
Elevated levels of IL-6 induce insulin resistance in both adipose cells [3] and the liver [4] by reducing the expression of a key protein involved in insulin signalling (insulin resistance substrate-1; IRS-1) in these cells. Furthermore, the insulin-regulated glucose-transporting molecules (GLUT-4) are also reduced in the adipose cells, thus extending the insulin resistance to both the signalling and effector compartments.
In contrast to the liver and adipose cells, there has been much controversy over the role of IL-6 in insulin signalling and action in skeletal muscle. Short-term infusion experiments have suggested positive effects [5], possibly due to the activation of AMP kinase activity—an important molecule for glucose and lipid oxidation and metabolism.
Recently, we studied the effect of chronically elevated IL-6 levels in mouse models by overexpressing this molecule in the skeletal muscle. This caused an increased secretion of IL-6 to the blood and chronically elevated systemic levels [6]. Interestingly, the mice lost body fat, consistent with previous demonstrations that IL-6 counteracts obesity development by increasing energy expenditure [7]. Our results agreed with this concept since food intake was not altered. More importantly, however, we also found the development of insulin resistance in the skeletal muscle through the reduction of insulin signalling (IRS-1) and glucose uptake (GLUT-4) [6]. These results corroborated previous findings as discussed above. However, a most remarkable result was that the livers of the mice were infiltrated with inflammatory cells [6], thus mimicking the condition of non-alcoholic steatohepatitis (NASH) in humans. Interestingly, this occurred in the absence of increased lipid infiltration in the liver cells, suggesting that inflammation may be induced through a mechanism that is differently regulated than lipid accumulation. Clearly, these factors are closely associated in human obesity, where both lipid accumulation and inflammation are seen. However, since the mice were not obese they had no increased lipid accumulation in the liver, which is a site of ectopic lipid storage.
Our results were both unexpected and intriguing. A potential scenario is that human obesity with insulin resistance, which is the typical intra-abdominal (visceral) obesity as discussed on this website, also leads to lipid accumulation in the liver cells (Figure). Lipid accumulation in the liver cells is, by itself, proinflammatory as we have recently found in experimental studies with human primary liver cells (under publication). However, the results with the animal model discussed above suggest that additional factors are required to move from the fairly benign condition of non-alcoholic fatty liver disease (NAFLD) to the clinically relevant NASH, with increased fibrosis formation and risk of liver cirrhosis. We are now postulating that increased IL-6 release by the adipose tissue in obesity plays a role.
Do we have other support for the potential role of IL-6 in the NAFLD conversion to NASH? This is where intra-abdominal obesity may be particularly interesting.
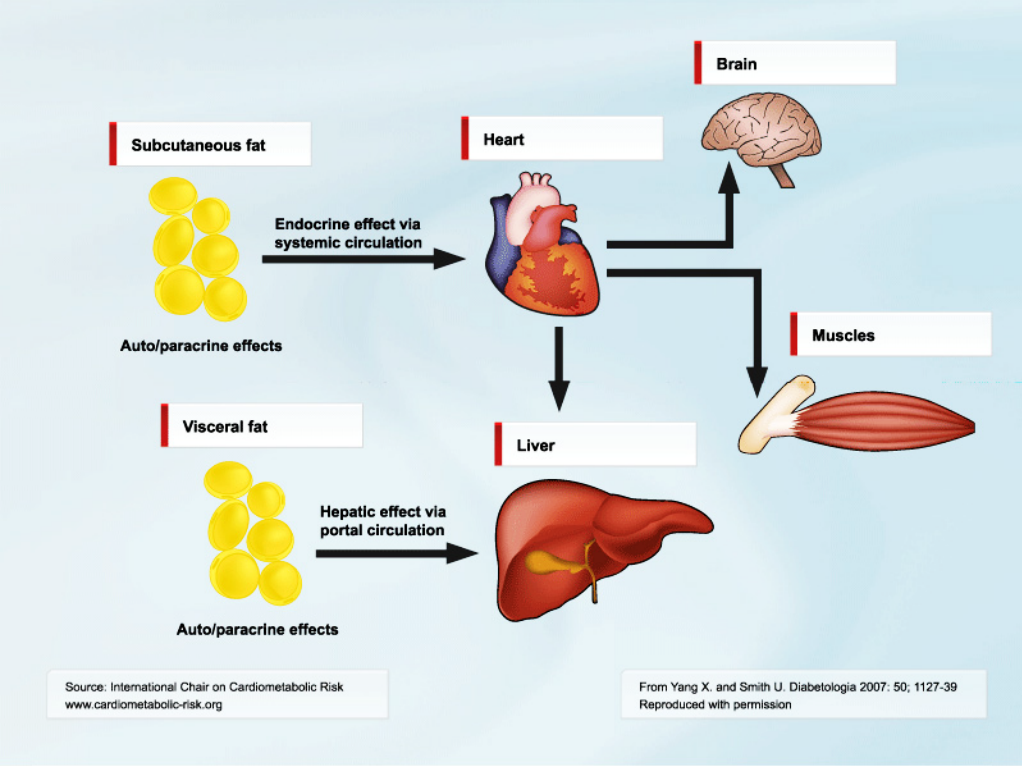
We know from several studies that intra-abdominal obesity also is associated with increased accumulation of liver fat and risk of NASH. Intra-abdominal fat is also highly proinflammatory, and a recent study where IL-6 was measured in portal blood showed that the levels were around 50% higher than in the peripheral circulation [8]. Després and colleagues also recently demonstrated that intra-abdominal/abdominal obesity was characterized by elevated circulating IL-6 levels [9]. Correlations between amount of intra-abdominal fat, liver fat and liver inflammation have also been documented. Thus, although more experimental and clinical work needs to be performed to prove these associations as causal, the scenario of obesity with insulin resistance, i.e., intra-abdominal/abdominal obesity, as a risk for NAFLD/NASH development seems highly appealing and supported by recent studies. In addition to a role in the infiltration of inflammatory cells in the liver, IL-6 may also have a negative effect on insulin sensitivity. Insulin resistance promotes a proinflammatory state since insulin by itself is anti-inflammatory and counteracts the effects of IL-6 [10].
In conclusion, intra-abdominal/abdominal obesity presents itself as a risk not only for cardiovascular disease but also for NAFLD/NASH. IL-6 seems a likely important molecule for these associations. Anti-IL-6 therapy has recently been introduced as a treatment for rheumatoid arthritis. Whether this concept can be extended to NAFLD/NASH needs to be tested.
References
- Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 1997; 82: 4196-200. PubMed ID: 9398739
- Sopasakis VR, Sandqvist M, Gustafson B, et al. High local concentrations and effects on differentiation implicate interleukin-6 as a paracrine regulator. Obes Res 2004; 12: 454-60. PubMed ID: 15044662
- Rotter V, Nagaev I and Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 2003; 278: 45777-84. PubMed ID: 12952969
- Senn JJ, Klover PJ, Nowak IA, et al. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002; 51: 3391-9. PubMed ID: 12453891
- Carey AL, Steinberg GR, Macaulay SL, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006; 55: 2688-97. PubMed ID: 17003332
- Franckhauser S, Elias I, Rotter Sopasakis V, et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia 2008; 51: 1306-16. PubMed ID: 18437347
- Wallenius V, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 2002; 8: 75-9. PubMed ID: 11786910
- Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007; 56: 1010-3. PubMed ID: 17287468
- Cartier A, Lemieux I, Alméras N, et al. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab 2008; 93: 1931-8. PubMed ID: 18319319
- Andersson CX, Sopasakis VR, Wallerstedt E, et al. Insulin antagonizes interleukin-6 signaling and is anti-inflammatory in 3T3-L1 adipocytes. J Biol Chem 2007; 282: 9430-5. PubMed ID: 17267401